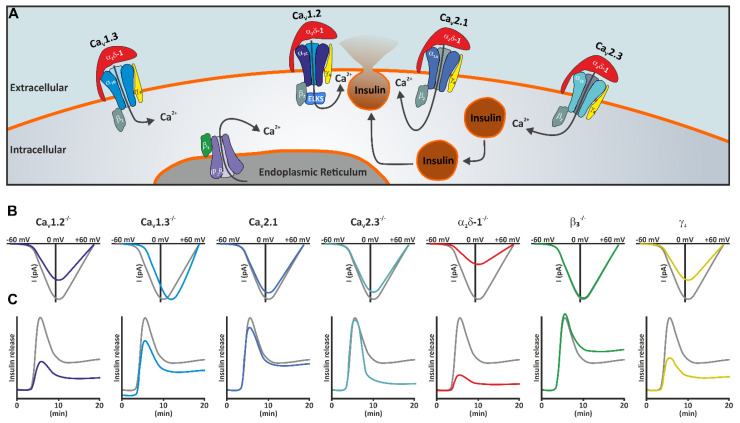
Figure 2.
Role of the HVCC subunits on pancreatic β-cell insulin release. (A). Localization of the HVCC isoforms in pancreatic β-cells with respect to insulin vesicles. At least in mouse, CaV1.2 channels are coupled to vesicle release, while evidence suggests that in human β-cells, CaV2.1 takes the central role. The localization of CaV1.2 channels at the release sites is controlled by the interaction between β2 subunit and ELKS. CaV2.3 channels are necessary for sustained insulin release, while the role of CaV1.3 is very controversial. β3 isoform does not come in complex with any HVCC but interacts with the IP3R. (B) Effect of genetic deletion or pharmacological block of each HVCC subunit isoform on total β-cell Ca2+ current. CaV1.2 contribute with ~45% of the whole-cell Ca2+ influx while CaV2.1 and CaV2.3 with ~20% each. CaV1.3 deletion only shifts the voltage dependence of activation. α2δ-1 deletion reduces all HVCC Ca2+ currents by ~70% while γ4 altered expression reduces only L-type Ca2+ currents (~70%). (C) Role of the HVCC subunits on GIIS. CaV1.2 deletion has the strongest effect as it is coupled to insulin vesicle release. CaV1.3 deletion reduces insulin secretion at lower glucose concentration while lack of CaV2.3 Ca2+ currents reduces second-phase GIIS. α2δ-1 deletion strongly reduces GIIS, while β3 deletion increases insulin release. γ4 reduced expression is expected to have similar effects as CaV1.2 deletion.