Abstract
Introduction:
Intestinal electrical stimulation (IES) has been reported to reduce body weight and improve glucose tolerance in obese and diabetic rats. Our study aimed to investigate possible IES mechanisms involving incretin hormones using intraduodenal glucose infusion in rats. We hypothesized that the enhanced release of postprandial glucagon-like peptide-1 (GLP-1) at early phase by IES was mediated through neuro/paracrine mechanisms involving the vagal nerve and glucose-dependent insulinotropic peptide (GIP).
Methods:
Fifteen normal male Sprague-Dawley rats chronically implanted with duodenal electrodes for IES and an intra-duodenum catheter for the infusion of glucose were studied in a series of sessions with IES of different parameters with and without atropine and M3 receptor antagonist. Blood samples were collected via the tail vein for the messurement of blood glucose, and plasma GLP-1 and GIP.
Results:
1) Compared to sham-IES, IES of 0.3ms reduced blood glucose by 16.5–28.4% between 30 and 120 min (all time points P<0.05), and IES of 3ms reduced blood glucose at 60 (12.6%) and 90 min (11.8%). IES of 0.3ms showed a greater hypoglycemic effect than 3ms (P=0.024) at 30 min. 2) IES elevated plasma GLP-1 with 0.3ms (P=0.001) and with 3ms (P=0.03). 3) IES substantially elevated plasma GIP with 0.3ms (p=0.002) and with 3ms (P<0.001) 4) Pretreatment of atropine and the M3 receptor antagonist 4-DAMP blocked the effects of IES on GLP-1, GIP and blood glucose.
Conclusions:
IES reduces postprandial blood glucose by enhancing the release of GLP-1 and GIP mediated via the cholinergic mechanism.
Keywords: Type 2 diabetes mellitus, Intestinal electrical stimulation, Incretin hormones, GLP-1, GIP
INTRODUCTIONS
Type 2 diabetes (T2D) is one of leading epidemics in human history closely associated with obesity [1]. The major basis for this link is the ability of obesity to engender insulin resistance which precedes the onset of type 2 diabetes and is generally considered to be an important factor in the pathogenesis of type 2 diabetes [2].
Although diabetes is primarily managed by lifestyle changes and dietary modifications, administration of a pharmacological agent is required especially when treatment goals are not achieved [3]. Incretin-based therapies have emerged that make use of the incretin system, which comprises the incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) that stimulate the release of insulin from pancreatic β cells at elevated glucose concentrations [4]. However, existing glucose-lowering agents may cause unwanted consequences, such as hypoglycemia and furthermore weight gain, an undesirable outcome with potential long-term adverse consequences on glycemic control itself [5].
Bariatric surgery is the most effective therapy for sustained weight loss in obese patients [6]. T2D remission after certain bariatric procedures, such as gastric bypass, can be observed within days after operation, even before any substantial weight loss occurs [7,8]. Accordingly, the accomplishment of rapid glycemic control is independent of weight loss [7,9]. While exact mechanisms involved in the hypoglycemic effect of gastric bypass remain elusive, the rapid improvement in glycemic control seems to be based on enhancing the endogenous action of incretin hormones by the bypass of the proximal small intestine allows a rapid delivery of nutrients to lower small intestine [9,10]. Bariatric surgery is typically limited to a small fraction of patients with morbid obesity due to invasiveness and procedure-induced complications [11,12].
Intestinal electrical stimulation (IES) has been reported to reduce body weight and improve glucose tolerance in obese and diabetic rats and is therefore proposed as a potential treatment for T2D [13–15]. Our previous studies showed that IES also increased GLP-1 after oral glucose intake [16]. However, oral ingestion of glucose involves gastric emptying, a compounding factor that may affect postprandial blood glucose level. To eliminate the effect of gastric emptying, we designed a series of experiments in this study to investigate the effects and mechanisms of IES on incretin hormones by directly perfusing glucose into the duodenum. The muscarinic acetylcholine receptor family consists of five subtypes, M1-M5. The muscarinic 3 (M3) receptor is one of the most important muscarinic acetylcholine subtype receptors; it mediates functions of intestinal smooth muscle contractions and plays a key role in mediating the physiological effects of vagal cholinergic activation[17].
The aims of this study were to investigate the hypoglycemic effects of acute IES on blood glucose and incretin hormones, GLP-1 and GIP after intraduodenal infusion of glucose and to explore mechanisms involving cholinergic pathways in rats.
MATERIALS AND METHODS
Animal
Fifteen male Sprague-Dawley rats (Charles River Laboratories, Kingston, NY), 385 ± 25g at the time of surgery, were used in this study. They were housed individually in animal facility under controlled temperature (22 ± 2°C), humidity (40%–50%) and fixed 12-h light-dark cycle. The surgical and experimental protocols of this study were approved by the Animal Resource Center at Johns Hopkins University Bayview Medical Center (Baltimore, MD).
Surgery for Intestinal Electrodes and Intraduodenal Catheter
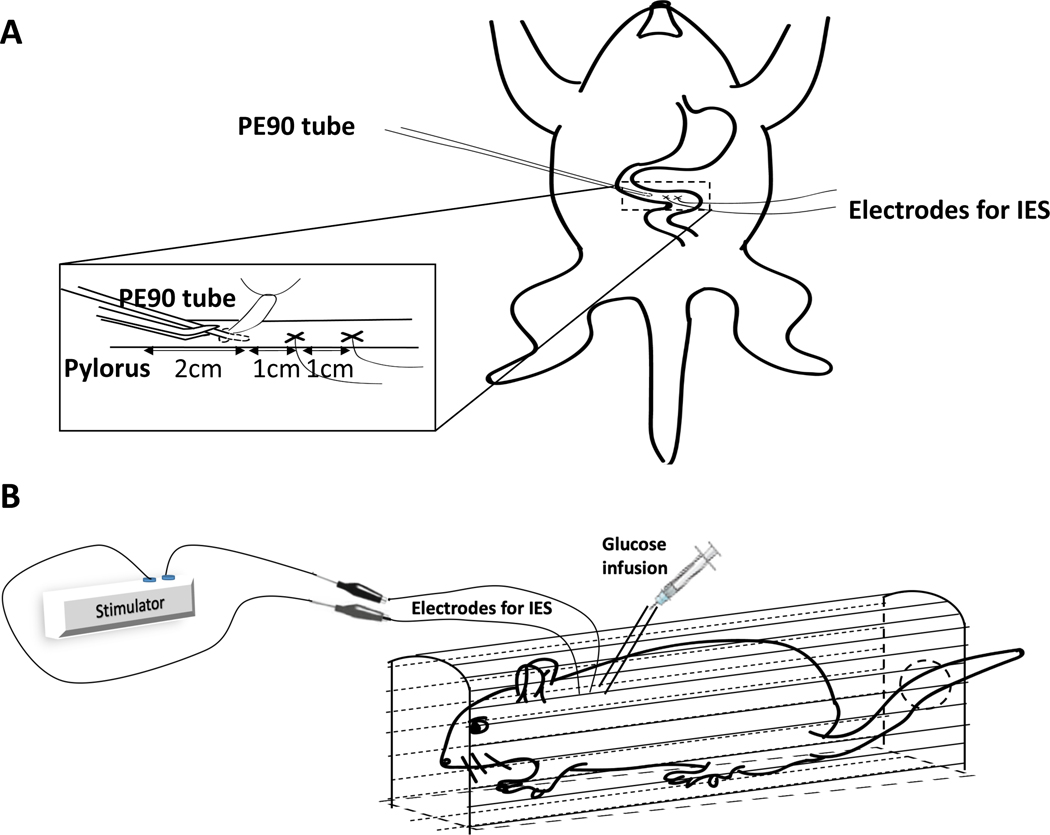
All surgical procedures were performed aseptically with rats under anesthesia with 12% isoflurane (Terrell, Piramal Critical Care, Inc., USA). Briefly, a middle-line incision (2–3cm) was made in the abdominal area to expose the small intestine. One pair of stainless-steel streamline cardiac pacing wires (Medtronic, Minneapolis, MN) was chronically placed at the duodenum serosa 3cm distal to the pylorus. The tip of the exposed wires was sutured on the serosal surface of the small intestine. The distance between two electrodes in a pair was about 1 cm. A polyethylene tubing (PE90) for glucose infusion was inserted into the duodenum at 2 cm beyond the pylorus through a small duodenal incision and fixed on the edge of the incision by a ligature. The connecting electrode wires and polyethylene tubing were passed through the subcutaneous layer of the abdomen to and externalized at the back of the neck (Fig. 1A). An antibiotic medication, cefazolin (20 mg/kg, i.p.), was given for three days to prevent infection. Buprenorphine with sustained release (1 mg/kg) was subcutaneously injected during the operation. Experiments were performed when the animals were completely recovered from surgery.
Figure 1.
A. Surgery for intestinal electrodes and intraduodenal catheter. One pair of stainless-steel streamline cardiac pacing wires was chronically placed at the duodenum serosa 3cm distal to the pylorus (the distance between two electrodes in a pair was about 1 cm) A polyethylene tubing (PE90) for glucose infusion was inserted into the duodenum at 2 cm beyond the pylorus through a small duodenal incision and fixed on the edge of the incision by a ligature. B. Performing IES with different pulse widths through stimulator during an intraduodenal glucose tolerant test. The rat was infused with glucose (2g/kg, 2ml) via the intraduodenal catheter for 10 minutes using a syringe pump and received IES treatments for 2 hours.
Intraduodenal glucose tolerance test (IDGTT)
An intraduodenal glucose tolerance test was performed as follows: After an overnight fast, the rat was infused with glucose (2g/kg, 2ml) via the intraduodenal catheter for 10 min using a syringe pump and received one of the treatments for 2 hours. Blood samples were collected via the tail vein at baseline and 15, 30, 60, 90, 120 min after the glucose infusion. The blood glucose level was assessed at each time point using a glucometer.
Experimental Protocols
Experiment 1:
Hypoglycemic effects IES with different pulse widths. This experiment aimed to investigate the effects of IES with different pulse widths in reducing blood glucose during an intraduodenal glucose tolerant test (Fig 1B). The rationale for this experiment was attributed to the fact that the effect of IES on intestinal smooth muscles was believed to be dependent on stimulation pulse width[16,18]. Three randomized IDGTT sessions were performed in the rats with following interventions: (1) IES with a pulse width of 0.3ms (IES-0.3ms), pulse frequency of 40Hz and amplitude of 2mA, the stimulation was on for 0.6s and off for 0.9s. The pulse width of 0.3ms was set to activate nerves (enteric nerve and autonomic nerve); the intermittent stimulation was designed to mimic the intrinsic intestinal slow waves that repeat at an interval of 1.5s (0.6s+0.9s=1.5s). (2) IES with a pulse width of 3ms (IES-3ms), other parameters same as these of IES-0.3ms. The pulse width of 3ms was previously reported to activate smooth muscle[19]. (3) Sham-IES: same experiment setup for stimulation with 0mA output. Experimental sessions were at least 3 days apart.
Experiment 2:
Role of atropine on hypoglycemic effect of IES with different pulse widths during IDGTT. A pulse width of 3ms with IES was previously shown to alter smooth muscle functions, whereas 0.3ms was believed to alter only nerve functions[16,18,19]. This experiment was to investigate the role of atropine on the inhibitory effect of IES on IDGTT. The rats were performed in 3 randomized IDGTT sessions with following interventions: (1) IES-0.3ms with atropine; (2) IES-3ms with atropine; (3) Sham-IES with atropine. Atropine (0.5mg/kg, Sigma-Aldrich, St. Louis, MO, USA) was intraperitoneally injected 10min before infusion. Experimental sessions were at least 3 days apart.
Experiment 3:
Role of 4-DAMP on effect of IES with different pulse widths during IDGTT. This experiment was designed to investigate the role of M3 receptor involved in the IES effects. 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP) is a selective muscarinic acetylcholine receptor (mAChR) M3 antagonist. The rats were performed in 3 sessions with following interventions: (1) IES-0.3ms with 4-DAMP; (2) IES-3ms with 4-DAMP; (3) Sham-IES with 4-DAMP. 4-DAMP (0.5mg/kg, Sigma-Aldrich, St. Louis, MO, USA) was intraperitoneally injected 10min before infusion. Experimental sessions were at least 3 days apart.
Measurements of incretin hormones
In Experiments 2 and 3, blood samples were collected at different time points (0, 15, 30, 60min) after infusion and placed in testing tubes containing chilled EDTA, aprotinin, and diprotin A (Sigma-Aldrich, St. Louis, MO, USA). Plasma GLP-1 and glucose-dependent insulinotropic peptide (GIP) were assessed using corresponding commercial ELISA kits (Sigma-Aldrich, St. Louis, MO, USA).
Statistical Analysis
All values are expressed as mean ± SE. Paired Student’s t test was used to compare the difference in blood glucose and the area under curve (AUC) of blood glucose levels between IES and control or Sham-IES. Two-way repeated measurement of analysis of variance was applied to investigate the difference in blood glucose, GLP-1, GIP in plasma among different groups. The post hoc Fisher’s test was used to compare the difference between two groups. P value of < 0.05 was considered significant.
RESULTS
IES decreased blood glucose after intraduodenal glucose infusion
IES with different pulse widths were examined if they had hypoglycemic effects during IDGTT. Compared to Sham-IES (Fig.2A), both IES reduced blood glucose levels after the intraduodenal glucose infusion. IES with 0.3ms reduced blood glucose from 30 min to 120 min (P < 0.05, vs. Sham-IES at all time points from 30 min); IES-3ms reduced blood glucose only at 60 min (P < 0.05 vs. Sham-IES) and 90 min (P < 0.05 vs. Sham-IES). At 30min after the IDGTT, IES with 0.3ms had a greater hypoglycemic effect than 3.0ms (P < 0.05, IES-0.3ms vs. IES-3.0ms). Compared to Sham-IES, both IES significantly reduced the AUC of the blood glucose level after the intraduodenal glucose infusion (P < 0.001 for IES-0.3ms; P < 0.05 for IES-3.0) (Fig.2B).
Figure 2.
IES decreased blood glucose after intraduodenal glucose infusion. A. The blood glucose during IDGTT. * p < 0.05, ** p < 0.01 vs. Sham-IES, # p < 0.05 vs. IES-3.0ms. B. AUC of blood glucose. * p < 0.05, # p < 0.001 vs. Sham-IES. The data was presented as the mean ± SEM, n = 8. IES, intestinal electrical stimulation; IDGTT, intraduodenal glucose tolerance test; AUC, area under curve; BG, blood glucose.
IES increased plasma GLP-1 and GIP after the intraduodenal glucose infusion
Plasma hormones GLP-1 and GIP were measured during IDGTT to assess the effects of IES on the release of these incretin hormones. As shown in Fig. 3A, IES significantly elevated plasma GLP-1 concentration the during IDGTT. After 15 minutes of infusion, IES with 0.3ms increased plasma GLP-1 levels compared to Sham-IES (P < 0.001 vs. Sham-IES), then the plasma GLP-1 concentration was decreased gradually from 30min to 60min (P = NS vs. Sham-IES). However, compared to Sham-IES, IES of 3ms increased plasma GLP-1 level from 15min to 60min (P < 0.05 vs. Sham-IES at 15min; P < 0.001 vs. Sham-IES at 30min; P < 0.05 vs. Sham-IES at 60min). Furthermore, GLP-1 in the IES with 3.0ms was significantly higher than that in the IES with 0.3ms at 60min (P < 0.05 vs. IES-0.3ms).
Figure 3.
IES increased plasma GLP-1 and GIP after the intraduodenal glucose infusion. A. The plasma GLP-1 level during IDGTT. * p < 0.05, # p < 0.001 vs. Sham-IES, & p < 0.05 vs. IES-0.3ms. B. The plasma GIP level during IDGTT. * p < 0.05, # p < 0.001 vs. Sham-IES, & p < 0.05 vs. IES-0.3ms. The data was presented as the mean ± SEM, n = 8. IES, intestinal electrical stimulation; IDGTT, intraduodenal glucose tolerance test.
Both of IES with 0.3ms and 3.0ms enhanced GIP secretion after the intraduodenal glucose infusion (Fig. 3B). Compared to Sham-IES, IES with 0.3ms increased plasma GIP from 15min to 30min (P < 0.05 vs. Sham-IES at 15min and 30min), and similar results were noticed in IES with 3.0ms (P < 0.05 vs. Sham-IES at 15min, P < 0.001 vs. Sham-IES at 30min). Furthermore, IES-3.0 enhanced GIP secretion compared to IES-0.3ms at 30min (P < 0.05 vs. IES-0.3ms).
Role of atropine in the IES-induced hypoglycemic effect
In order to investigate the cholinergic mechanism of the IES-induced hypoglycemic effect, the muscarinic cholinergic receptor antagonist atropine was used in this experiment. Atropine completely blocked the effect of IES on blood glucose. As shown in Fig.4, there were no changes in Sham-IES with atropine compared to Sham-IES from 15min to 120min. Atropine did not alter blood glucose (P = NS, Sham-IES vs. Sham-IES + Atropine for all time points and AUC of blood glucose). However, hypoglycemic effect of IES-0.3ms was blocked by atropine: at the presence of atropine, no difference was noted between IES-0.3ms and Sham-IES (P = NS, Sham-IES + Atropine vs. IES-0.3ms+atropine, in Fig 4A and Fig 4B). Similarly, atropine also blocked the effect of IES-3.0ms: after atropine administration, no differences were noted between IES-3.0ms and Sham-IES for all time points and AUC of blood glucose (P = NS, Sham-IES+atropine vs. IES-3.0ms+atropine, in Fig 4A and Fig 4B).
Figure 4.
Role of atropine in the hypoglycemic effect of IES. A. The blood glucose during IDGTT. B. AUC of blood glucose. The data was presented as the mean ± SEM, n = 8. IES, intestinal electrical stimulation; IDGTT, intraduodenal glucose tolerance test; AUC, area under curve; BG, blood glucose.
Role of atropine in the IES-induced increase in plasma GLP-1 and GIP
Atropine itself suppressed the plasma GLP-1 secretion and blocked the effects of IES on GLP-1 and GIP secretion. After atropine administration, the plasma GLP-1 concentration in Sham-IES decreased compared to without atropine from 15min to 30min (P < 0.05 Sham-IES+atropine vs. Sham-IES, Fig 5A, B). As shown in Fig. 5A, the plasma GLP-1 in IES-0.3ms with atropine were lower than Sham-IES at 30min after infusion (P < 0.05 vs. Sham-IES). The effects of IES-0.3ms on GLP-1 secretion was blocked by atropine. Compared with IES-0.3ms, IES-0.3ms+atropine decreased plasma GLP-1 at 15min and 30min during IDGTT (P < 0.05, vs. IES-0.3ms, Fig 4A). As shown in Fig. 5B, the plasma GLP-1 in IES-3.0ms+atropine was lower than Sham-IES from 15min to 30min after infusion (P < 0.05 vs. Sham-IES). The effects of IES-3.0ms IES on plasma GLP-1 also was blocked by atropine. IES-3.0ms+atropine decreased plasma GLP-1 compared to IES-3.0ms from 15min to 60min during IDGTT (P < 0.05, vs. IES-0.3ms, Fig 5B).
Figure 5.
Role of atropine in the IES-induced increase in plasma GLP-1 and GIP. A. Effects of atropine on plasma GLP-1 level in IES-0.3ms during IDGTT. * p < 0.05 vs. Sham-IES, # p < 0.05 vs. IES-0.3ms. B. Effects of atropine on plasma GLP-1 level in IES-3.0ms during IDGTT. * p < 0.05 vs. Sham-IES, # p < 0.05 vs. IES-3.0ms. C. Effects of atropine on plasma GIP level in IES-0.3ms during IDGTT. * p < 0.05 vs. Sham-IES, # p < 0.05 vs. IES-0.3ms. D. Effects of atropine on plasma GIP level in IES-3.0ms during IDGTT. * p < 0.05 vs. Sham-IES, ** p < 0.001 vs. Sham-IES, # p < 0.05 vs. IES-0.3ms, ## p < 0.001 vs. IES-0.3ms. The data was presented as the mean ± SEM, n = 8. IES, intestinal electrical stimulation; IDGTT, intraduodenal glucose tolerance test.
After atropine administration, no difference of the plasma GIP was noted in Sham-IES with atropine compared to Sham-IES from 15min to 60min (P = NS, Sham-IES + atropine vs. Sham-IES, Fig 5C, D). The effect of IES-0.3ms was blocked by atropine: no difference was noted between IES-0.3ms with atropine and Sham-IES (P = NS, IES-0.3ms+atropine vs Sham-IES., in Fig 5C) and IES-0.3ms with atropine decreased plasma GIP concentration compared to IES-0.3ms at 15min (P < 0.05, vs. IES-0.3ms, in Fig 5C). The effect of IES-3.0ms was also blocked by atropine: there were no changes in IES-3.0ms with atropine compared to Sham-IES from 15min to 60min (P = NS, IES-3.0ms+atropine vs Sham-IES, in Fig 5D) and IES-3.0ms with atropine decreased plasma GLP-1 compared to IES-3.0ms from 15min to 60min (P < 0.05, vs. IES-3.0ms, in Fig 5D).
Role of the M3 antagonist in the hypoglycemic effect of IES
Pretreatment of M3 antagonist decreased blood glucose after intraduodenal glucose infusion and blocked the inhibitory effect of IES on blood glucose. As shown in Fig. 6A, Sham-IES with 4-DAMP significantly decreased the blood glucose level compared to Sham-IES from 60min to 90min (P < 0.05 vs. Sham-IES). Significantly lower blood glucose in IES-0.3ms with 4-DAMP compared to Sham-IES from 30min to120min (P < 0.05 vs. Sham-IES in 30min and 120min, P < 0.001 vs. Sham-IES in 60min and 90min). The decrease of blood glucose in IES-3.0ms with 4-DAMP was also noticed compared to Sham-IES from 60min to120min (P < 0.05 vs. Sham-IES). There is no obvious difference between the Sham-IES with 4-DAMP, IES-0.3ms with 4-DAMP and IES-3.0ms with 4-DAMP. Compared to Sham-IES, the Sham-IES with 4-DAMP, IES-0.3ms with 4-DAMP and IES-3.0ms with 4-DAMP significantly reduced the AUC of the blood glucose level after the intraduodenal glucose infusion (P < 0.001 for IES-0.3ms+4-DAMP; P < 0.05 for IES-3.0ms+4-DAMP and Sham-IES+4-DAMP) (Fig.6B).
Figure 6.
4-DAMP decreased blood glucose after intraduodenal glucose infusion. A. The blood glucose during IDGTT. * p < 0.05, ** p < 0.01 vs. Sham-IES. B. AUC of blood glucose. * p < 0.05, # p < 0.001 vs. Sham-IES. The data was presented as the mean ± SEM, n = 8. IES, intestinal electrical stimulation; IDGTT, intraduodenal glucose tolerance test; AUC, area under curve; BG, blood glucose.
Role of the M3 antagonist in the IES-induced increase in plasma GLP-1 and GIP
4-DAMP suppressed the plasma GLP-1 secretion and blocked the effects of IES on GLP-1. After 4-DAMP administration, the plasma GLP-1 concentration in Sham-IES decreased compared to without 4-DAMP from 15min to 60min (P < 0.05 Sham-IES+4-DAMP vs. Sham-IES, Fig 7A, B). As shown in Fig. 7A, the plasma GLP-1 in IES-0.3ms with 4-DAMP were also lower than Sham-IES from 15min to 60min (P < 0.05 vs. Sham-IES at all time points from 15min). The effects of IES-0.3ms on GLP-1 secretion was blocked by 4-DAMP. Compared with IES-0.3ms, IES-0.3ms+4-DAMP decreased plasma GLP-1 at 15min and 30min during IDGTT (P < 0.05, vs. IES-0.3ms, Fig 7A). As shown in Fig. 7B, the plasma GLP-1 in IES-3.0ms+4-DAMP was lower than Sham-IES from 15min to 60min after infusion (P < 0.05 vs. Sham-IES at all time points from 15min). The effects of IES-3.0ms IES on plasma GLP-1 also was blocked by 4-DAMP. IES-3.0ms+4-DAMP decreased plasma GLP-1 compared to IES-3.0ms from 15min to 60min during IDGTT (P < 0.05, vs. IES-3.0ms, Fig 7B).
Figure 7.
Role of the M3 antagonist in the IES-induced increase in plasma GLP-1 and GIP. A. Effects of 4-DAMP on plasma GLP-1 level in IES-0.3ms during IDGTT. *p < 0.05 vs. Sham-IES, # p < 0.05 vs. IES-0.3ms, & vs. Sham-IES+4DAMP. B. Effects of 4-DAMP on plasma GLP-1 level in IES-3.0ms during IDGTT. *p < 0.05 vs. Sham-IES, # p < 0.05 vs. IES-0.3ms, & vs. Sham-IES+4DAMP. C. Effects of 4-DAMP on plasma GIP level in IES-0.3ms during IDGTT. *p < 0.05 vs. Sham-IES. D. Effects of 4-DAMP on plasma GIP level in IES-3.0ms during IDGTT. *p < 0.05 vs. Sham-IES, # p < 0.05 vs. IES-0.3ms. The data was presented as the mean ± SEM, n = 8. IES, intestinal electrical stimulation; IDGTT, intraduodenal glucose tolerance test.
4-DAMP promoted the release of plasma GIP but blocked the promotive effect of IES on GIP. After 4-DAMP administration, the plasma GIP concentration in Sham-IES increased compared to without 4-DAMP from 15min to 30min (P < 0.05 Sham-IES+4-DAMP vs. Sham-IES, Fig 7C, D). As shown in Fig. 7C, IES-0.3ms with 4-DAMP also increased plasma GIP compared to Sham-IES from 15min to 30min (P < 0.05 vs. Sham-IES at all time points from 15min). No difference was noted between IES-0.3ms with 4-DAMP and Sham-IES with 4-DAMP. There were also no changes in IES-0.3ms with 4-DAMP compared to IES-0.3ms from 15min to 60min. As shown in Fig. 7D, IES-3.0ms with 4-DAMP also increased plasma GIP compared to Sham-IES at 15min (P < 0.05 vs. Sham-IES). No difference was noted between IES-3.0ms with 4-DAMP and IES-3.0ms at 15min, but IES-3.0ms with 4-DAMP decreased the plasma GIP compared to IES-3.0ms at 30min (P < 0.05 vs. IES-3.0ms, in Fig 7D).
DISCUSSION
In the present study, we found that IES with both short and wide pulse widths reduced blood glucose levels following intraduodenal glucose infusion. Moreover, IES increased both plasma GLP-1 and GIP levels and the effects were blocked by atropine, suggesting a cholinergic mechanism. M3 antagonist, 4-DAMP decreased blood glucose after intraduodenal glucose infusion and also blocked the hypoglycemic effects of IES. Lastly, the M3 antagonist lowered plasma GLP-1 but increased plasma GIP, and blocked the secretive effects of IES on both GLP-1 and GIP (see Fig.8).
Figure 8.
Hypothetical mechanisms involved in the hypoglycemic effect of IES. IES activated duodenal K cells to enhance the realse of GIP-1 and enhanced GLP-1 release by accelerating intestinal transit; the action of these incretin hormones resulted in an increased insulin release and a decrease in postprandial blood glucose. These effects were blocked by atropine and M3 receptor antagonist. IES: intestinal electrical stimulation; GLP-1: glucagon-like peptide-1; 4-DAMP: 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide.
Originally developed for the treatment of gastrointestinal motility diseases such as pseudo-intestinal obstruction and postoperative intestinal pseudo-obstruction, IES has been reported to delay gastric emptying, accelerate intestinal transit and reduce food intake and nutrient absorption[15,16,20]. Recently we have discovered that in addition to its effects on gastrointestinal motility, IES exerts an inhibitory effect on postprandial blood glucose in obese and diabetic rats as well as healthy volunteers[21–23]. Further studies have demonstrated role of GLP-1 in the glucose-lowering effect of IES[23]. The unique aspects of the present study included 1) glucose was directly infused to the duodenum and thus eliminated the compounding effect of gastric emptying; 2) for the first time the effect of IES on GIP was investigated and 3) the role of cholinergic nerve in the effects of IES on incretin hormones was studied.
GLP-1 and GIP are widely recognized incretin hormones that account for up to 70% of the total insulin secretory response after a meal: oral ingestion of glucose triggers more insulin release than a similar plasma glucose profile delivered intravenously[24–28]. The role of GLP-1 in the hypoglycemic effect of IES was indicated in a previous where Exendin (9–39) as a GLP-1 antagonist partly blocked the hypoglycemic effect of IES[23]. Electrical stimulation of the duodenum was reported to increase the GLP-1 level in the presence of nutrients in anesthetized rats[29]. In the present study, the experiment was performed in conscious rats and the glucose was infused directly into the duodenum via the chronically implanted intraduodenal tube so that the effect of gastric emptying was eliminated. We found that both IES-0.3ms and IES-3ms enhanced the secretion of GLP-1 at 15 min following the intraduodenal glucose infusion: the time frame when the nutrient did not reach the distal ileum. Although some papers showed that the density of L-cells in the human duodenum may be sufficiently high to account for the early phase of GLP-1 secretion[30], the secretion of GLP-1 at 15 min was believed to be regulated by a neural or hormonal proximal-distal loop, involving the relay of information about nutrient from the proximal duodenum to the L cells in the distal ileum[26].
Incretin hormone GIP, secreted from the K cells located in the duodenum in response to nutrient intake to potentiate insulin release from β-cells in healthy humans[31], is also considered as an endocrine mediator of this proximal-distal loop in rats[26]. In one study, while GIP was increased over baseline in response to nutrient infusion, electrical stimulation of the duodenum showed no further effect on the increase in GIP in anesthetized rats[29]. In the present study, however, both IES of 0.3 and 3ms significantly enhanced the glucose-induced increase in plasma GIP. The increased GIP may act through the nervous system (either vagal or myenteric) to indirectly stimulate the secretion of GLP-1 and/or act directly at the level of the L cell. It’s unknown if the enteroendocrine K cell is also electrical excitable in addition to its chemosensing to luminal nutrients. Using the techniques of transgenic mice and primary culture, glucose-triggered GIP secretion was found to be SGLT1-dependent and modulated by KATP channel activity but not determined by sweet taste receptors[32]. Interestingly, the postprandial rise of GIP enhanced by IES was blocked by the pretreatment of atropine in this study, indicating an involving of vagal pathway in the regulatory effect of IES on GIP secretion.
More importantly, at 60 min, the plasma GLP-1 remained high in the rats treated with IES of 3ms but returned to baseline in the rats with sham IES or IES of 0.3 ms. This elevated plasma GLP-1 level with IES of 3m was speculated to be attributed to the accelerative effect of IES of 3m on intestinal transit, which brought more nutrients to the distal ileum and resulted in release of GLP-1 from L-cells in the distal ileum[16]. Since M3 receptor plays an important role in the regulation of gastrointestinal motility in normal and diabetes[37], the effect of 4-DAMP on IES enhanced GLP-1 release was explored. Pretreatment of 4-DAMP blocked the enhanced release of GLP-1 at the second phase of postprandial state by IES of 3ms, which is believed to be able to accelerate small intestinal transit [21,38,39]. There was a limitation in this study: the small intestinal transit was not measured so it’s unclear if IES accelerate the small intestinal transit which brings the nutrient to the distal ileum to stimulate the release of GLP-1 from L-cell. Furthermore, the hypoglycemic effect of 4-DAMP may partly due to the predominant presence of M3 subtype receptor in pancreatic β-cells[40,41], which reduces the glucose-induced secretion of GLP-1 and GIP.
In addition to the enhance release of GLP-1by IES via enhance the release of GIP which directly acting on L-cell to release GLP-1 or activate vagal afferent pathway to indirectly, IES was reported to modulate neuronal activities in the nucleus of the solitary tract and hypothalamus in rats via the activation of vagal afferent pathway[42,43]. The activated vagal afferent may stimulate the effect celiac vagal to distal ileum to release GLP-1[26].
In conclusion, IES reduces postprandial blood glucose by enhancing the release of GLP-1 and GIP mediated via the cholinergic mechanism (summary in Fig 8).
Acknowledgments
Source of funding: Supported by a grant from NIH: R01DK107754
Footnotes
Conflict of Interest: All authors have no conflict of interest
Ethical Statement
All procedures performed in studies involving animals were in accordance with the ethical standards of the institutional and/or national research committee. This article does not contain any studies with human performed by any of the authors.
References:
- 1.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat Rev Endocrinol. 2011November8;8(4):228–36. [DOI] [PubMed] [Google Scholar]
- 2.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000August;106(4):473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tasyurek HM, Altunbas HA, Balci MK, Sanlioglu S. Incretins: their physiology and application in the treatment of diabetes mellitus. Diabetes Metab Res Rev. 2014July;30(5):354–71. [DOI] [PubMed] [Google Scholar]
- 4.Nauck M Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016March;18(3):203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maleckas A, Venclauskas L, Wallenius V, Lönroth H, Fändriks L. Surgery in the treatment of type 2 diabetes mellitus. Scand J Surg SJS Off Organ Finn Surg Soc Scand Surg Soc. 2015March;104(1):40–7. [DOI] [PubMed] [Google Scholar]
- 6.De Luca M, Angrisani L, Himpens J, Busetto L, Scopinaro N, Weiner R, et al. Indications for Surgery for Obesity and Weight-Related Diseases: Position Statements from the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO). Obes Surg. 2016;26(8):1659–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thaler JP, Cummings DE. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009June;150(6):2518–25. [DOI] [PubMed] [Google Scholar]
- 8.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005April;15(4):474–81. [DOI] [PubMed] [Google Scholar]
- 9.Vetter ML, Cardillo S, Rickels MR, Iqbal N. Narrative review: effect of bariatric surgery on type 2 diabetes mellitus. Ann Intern Med. 2009January20;150(2):94–103. [DOI] [PubMed] [Google Scholar]
- 10.Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005January;90(1):359–65. [DOI] [PubMed] [Google Scholar]
- 11.Sagar PM. Surgical treatment of morbid obesity. Br J Surg. 1995June;82(6):732–9. [DOI] [PubMed] [Google Scholar]
- 12.Crookes PF. Surgical treatment of morbid obesity. Annu Rev Med. 2006;57:243–64. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Chen J. Intestinal electric stimulation decreases fat absorption in rats: therapeutic potential for obesity. Obes Res. 2004August;12(8):1235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aberle J, Busch P, Veigel J, Duprée A, Roesch T, zu Eulenburg C, et al. Duodenal Electric Stimulation: Results of a First-in-Man Study. Obes Surg. 2016February;26(2):369–75. [DOI] [PubMed] [Google Scholar]
- 15.Yin J, Zhang J, Chen JDZ. Inhibitory effects of intestinal electrical stimulation on food intake, weight loss and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol. 2007July;293(1):R78–82. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Chen JDZ. Pulse Width-Dependent Effects of Intestinal Electrical Stimulation for Obesity: Role of Gastrointestinal Motility and Hormones. Obes Surg. 2017;27(1):70–7. [DOI] [PubMed] [Google Scholar]
- 17.Gautam D, Gavrilova O, Jeon J, Pack S, Jou W, Cui Y, et al. Beneficial metabolic effects of M3 muscarinic acetylcholine receptor deficiency. Cell Metab. 2006November;4(5):363–75 [DOI] [PubMed] [Google Scholar]
- 18.Chen JDZ, Yin J, Wei W. Electrical therapies for gastrointestinal motility disorders. Expert Rev Gastroenterol Hepatol. 2017May;11(5):407–18. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Chen JDZ. Cellular effects of gastric electrical stimulation on antral smooth muscle cells in rats. Am J Physiol Regul Integr Comp Physiol. 2010June;298(6):R1580–1587. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Chen J. Intestinal Electric Stimulation Decreases Fat Absorption in Rats: Therapeutic Potential for Obesity. Obes Res. 2004August;12(8):1235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Zhu W, Zhang S, Chen JDZ. Chronic intestinal electrical stimulation improves glucose intolerance and insulin resistance in diet-induced obesity rats. Obes Silver Spring Md. 2017;25(6):1061–8. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Xiang Y, Qiao X, Dai Y, Chen JDZ. Hypoglycemic effects of intraluminal intestinal electrical stimulation in healthy volunteers. Obes Surg. 2011February;21(2):224–30. [DOI] [PubMed] [Google Scholar]
- 23.Ouyang X, Li S, Tan Y, Lin L, Yin J, Chen JDZ. Intestinal electrical stimulation attenuates hyperglycemia and prevents loss of pancreatic β cells in type 2 diabetic Goto-Kakizaki rats. Nutr Diabetes. 201906;9(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol. 2009January1;587(Pt 1):27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anini Y, Fu-Cheng X, Cuber JC, Kervran A, Chariot J, Roz C. Comparison of the postprandial release of peptide YY and proglucagon-derived peptides in the rat. Pflugers Arch. 1999August;438(3):299–306. [DOI] [PubMed] [Google Scholar]
- 26.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999April;140(4):1687–94. [DOI] [PubMed] [Google Scholar]
- 27.Gutzwiller JP, Göke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999January;44(1):81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007May;132(6):2131–57. [DOI] [PubMed] [Google Scholar]
- 29.Sandoval D, Dunki-Jacobs A, Sorrell J, Seeley RJ, D’Alessio DD. Impact of intestinal electrical stimulation on nutrient-induced GLP-1 secretion in vivo. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2013August;25(8):700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, et al. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab. 2006March;290(3):E550–559. [DOI] [PubMed] [Google Scholar]
- 31.Christensen MB. Glucose-dependent insulinotropic polypeptide: effects on insulin and glucagon secretion in humans. Dan Med J. 2016April;63(4). [PubMed] [Google Scholar]
- 32.Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia. 2009February;52(2):289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.. Rask E, Olsson T, Söderberg S, Johnson O, Seckl J, Holst JJ, et al. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care. 2001. September;24(9):1640–5. [DOI] [PubMed] [Google Scholar]
- 34.. Anini Y, Hansotia T, Brubaker PL. Muscarinic receptors control postprandial release of glucagon-like peptide-1: in vivo and in vitro studies in rats. Endocrinology. 2002. June;143(6):2420–6. [DOI] [PubMed] [Google Scholar]
- 35.. Abello J, Ye F, Bosshard A, Bernard C, Cuber JC, Chayvialle JA. Stimulation of glucagon-like peptide-1 secretion by muscarinic agonist in a murine intestinal endocrine cell line. Endocrinology. 1994. May;134(5):2011–7. [DOI] [PubMed] [Google Scholar]
- 36.. Anini Y, Brubaker PL. Muscarinic receptors control glucagon-like peptide 1 secretion by human endocrine L cells. Endocrinology. 2003. July;144(7):3244–50. [DOI] [PubMed] [Google Scholar]
- 37.Cellini J, Jukic AMZ, LePard KJ. Neostigmine-induced contraction and nitric oxide-induced relaxation of isolated ileum from STZ diabetic guinea pigs. Auton Neurosci Basic Clin. 2011December7;165(2):178–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu X, Lei Y, Chen JDZ. Duodenum electrical stimulation delays gastric emptying, reduces food intake and accelerates small bowel transit in pigs. Obes Silver Spring Md. 2011February;19(2):442–8. [DOI] [PubMed] [Google Scholar]
- 39.Wang WF, Yin JY, De Dz Chen J. Acceleration of small bowel transit in a canine hypermotility model with intestinal electrical stimulation. J Dig Dis. 2015March;16(3):135–42. [DOI] [PubMed] [Google Scholar]
- 40.Gautam D, Han S-J, Hamdan FF, Jeon J, Li B, Li JH, et al. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006June;3(6):449–61. [DOI] [PubMed] [Google Scholar]
- 41.Ito Y, Kaji M, Sakamoto E, Terauchi Y. The beneficial effects of a muscarinic agonist on pancreatic β-cells. Sci Rep. 201907;9(1):16180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Zhu H, Chen JDZ. Central neuronal mechanisms of intestinal electrical stimulation: effects on duodenum distention-responsive (DD-R) neurons in the VMH of rats. Neurosci Lett. 2009June19;457(1):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun Y, Qin C, Foreman RD, Chen JDZ. Intestinal electric stimulation modulates neuronal activity in the nucleus of the solitary tract in rats. Neurosci Lett. 2005September2;385(1):64–9. [DOI] [PubMed] [Google Scholar]