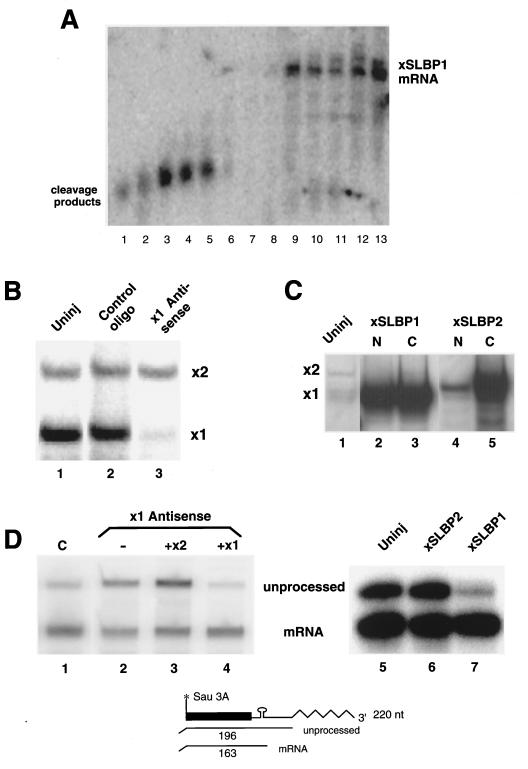
FIG. 4.
SLBP1, but not SLBP2, is involved in histone pre-mRNA processing. (A) A series of antisense oligonucleotides (AX07 to AX12, lanes 1 to 6; AX01 to AX06, lanes 7 to 12) directed against SLBP1 mRNA were injected into Xenopus oocytes, and RNA was prepared 4 h later. The RNA was resolved by electrophoresis on a 0.8% agarose gel and probed for SLBP1 mRNA. (B) Oocytes were injected with oligonucleotide AX09 and a control oligonucleotide, and extracts were prepared 48 h later. The extracts were assayed by mobility shift assay for the formation of complexes that bind to the stem-loop. (C) Oocytes were injected with synthetic mRNAs encoding SLBP1 (lanes 2 and 3) or SLBP2 (lane 3 and 4). Forty-eight hours later, the oocytes were fractionated into nuclei and cytoplasm and assayed for the presence of complexes that bound the stem-loop. Lane 1 shows an extract from uninjected oocytes. Extracts from one oocyte were analyzed in each lane. (D) Oocytes were injected with oligonucleotide AX09 (lanes 2 to 4) or with a control oligonucleotide (lane 1). Four hours later, synthetic mRNA encoding SLBP2 (lane 3) or SLBP1 (lane 4) was injected into some of the oocytes. After 48 h, histone H2a-614 DNA was injected into the nucleus. RNA was prepared 18 h after the injection of H2a-614 DNA, and the transcripts were assayed for processed and unprocessed histone mRNA by using an S1 nuclease assay, as shown at the bottom. In a separate experiment, untreated oocytes were injected with buffer (lane 5) or mRNAs encoding frog SLBP2 (lane 6) or frog SLBP1 (lane 7) 48 h prior to injection of histone H2a-614 DNA into the nucleus. RNA was prepared 18 h after injection of the DNA and analyzed by using S1 nuclease mapping.