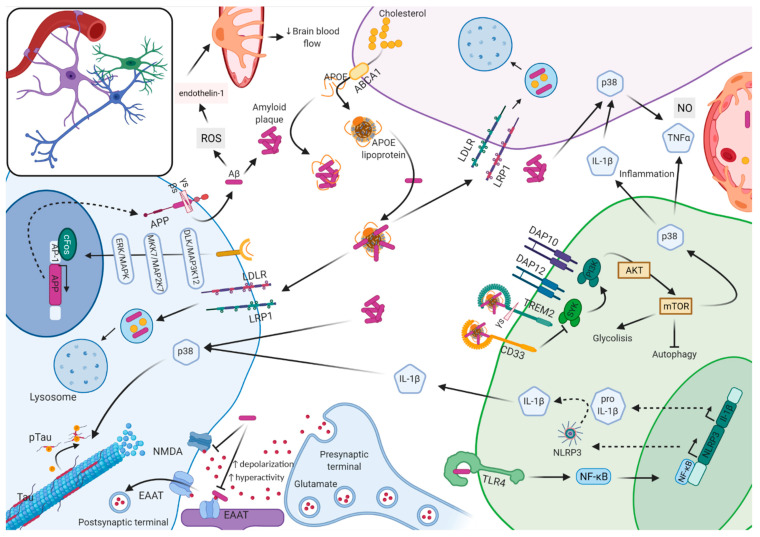
Figure 1.
Schematic representation of the molecular interplay between neurons, astrocytes, microglia, and vasculature system in Alzheimer’s disease. Neurons produce the transmembrane amyloid precursor protein (APP). APP is cleaved by γ (γs) and β secretase (βs) into amyloid β (Aβ) units of different length aggregates extracellularly into plaques. Oligomeric Aβ promotes the generation of ROS that triggers the release of endothelin-1, causing perycite constriction, which decreases brain blood flow. Soluble Aβ blocks the reuptake of synaptically released glutamate by either N-methyl-D-aspartate receptor (NMDA) or by excitatory amino acid transporter (EAAT) receptors causing glutamate accumulation perisynaptically (excitotoxicity), which increases depolarization and promotes hyperactivity. In microglia, Aβ binds to the toll-like receptor 4 (TLR4), which causes translocation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) from the cytosol to the nucleus, where it increases the transcription of NLRP3 and pro-IL-1β. In the cytoplasm, via activated caspase-1, the inflammasome promotes the maturation of IL-1β. Amyloid plaques stimulate the activation of p38MAPK (p38) in microglia, astrocyte, and neuron. In microglia, p38 activation results in upregulation of proinflammatory cytokines, interleukin 1β (IL-1β) and tumor necrosis factor α (TNFα); IL-1β in turns activates p38 in astrocytes and neurons. In astrocytes, p38 activation causes increased expression of TNFα and nitric oxide (NO) and excitotoxicity. In neurons, p38 activation results in tau phosphorylation (Tau → p-Tau) and microtubule disassembly. APOE is mostly generated by astrocytes; free APOE can facilitate Aβ blood bran barrier (BBB) transit, but it can also accelerate aggregation and deposition of Aβ in an isoform-dependent manner. APOE can be lipidated by ABCA1 transporter-forming lipoprotein particles that bind soluble Aβ, which are then uptaken by neurons and glia via cell-surface receptors, including low-density lipoprotein receptor (LDLR) and low-density lipoprotein receptor-related protein (LRP1), and degraded at the lysosome. When free APOE binds to ApoE receptors in neurons, it can activate a non-canonical MAPK pathway, in an isoform-dependent manner, that induces cFOS phosphorylation stimulating the transcription factor AP-1, which in turn enhances transcription of APP. The complex amyloid plaque lipidated APOE can stimulate microglia through transmembrane proteins triggering receptor expressed on myeloid cells 2 (TREM2) and sialic acid-binding Ig-like lectin 3 (CD33). TREM2 activation induces the association of TREM2 to DAP12, which gets phosphorylated and recruits spleen tyrosine kinase (SYK), which activates phosphoinositide 3-kinase (PI3K) that depends on DAP10. PI3K targets protein kinase B (AKT) and activates the mammalian target of rapamycin (mTOR), which activates glycolysis, the p38MAPK pathway, and inhibits autophagy. Instead, CD33 activation inhibits PI3K. The complement receptor 1 (CR1) is a receptor for the complement components C3b and C4b and promotes the phagocytosis of Aβ.