Abstract
Background
A positive link between periodontitis and chronic systemic disease has been indicated. However, few studies focused on the loss of teeth. Our analysis aims to analyze the relationship of periodontitis and number of teeth with the risk of coronary heart disease (CHD).
Material/Methods
A meta-analysis was conducted on qualified data extracted from the PubMed, Embase, and Cochrane Library databases. Only cohort studies were included in this study. We screened articles that assessed the periodontal condition and teeth number as well as the incidence or mortality of CHD. Hazard ratio (HR) and relative risk (RR) were calculated by Stata SE software.
Results
A total of 11 prospective studies with over 200 000 total participants were analyzed. Ten studies reported on periodontitis and CHD, and 4 studies included data on number of teeth. After adjusting for multivariate factors, there was a significant association between periodontitis and the risk of CHD (RR, 1.18; 95% confidence interval [CI], 1.10–1.26); the RR of CHD in the edentulous population was 1.20 (95% CI, 1.08–1.34). Moreover, results on the RR values for number of teeth were as follows: 24–17 teeth (RR, 1.12; 95% CI, 1.05–1.19); 16–11 (RR, 1.28; 95% CI, 1.15–1.42); and ≤10 (RR, 1.55; 95% CI, 1.43–1.69).
Conclusions
Our study showed that periodontitis is a risk factor for CHD and that the number of removed teeth is positively correlated with the risk of CHD. During clinical assessment, both factors need to be considered as factors associated with cardiovascular risks.
Keywords: Coronary Disease, Meta-Analysis, Periodontitis, Risk Factors
Background
Periodontal disease is defined as an inflammatory disease that affects the tissues surrounding the teeth. Periodontal disease is highly prevalent among adolescents and adults as well as older people, and its incidence is influenced by several modifiable risk factors, such as smoking, poor oral hygiene, and diabetes mellitus [1]. In 1993, results of a longitudinal study showed that periodontitis can be associated with coronary heart disease (CHD) [2]. Since then, many reports have shown an association between periodontitis and CHD, including cross-sectional [3], case-control [4], and cohort studies [5]. Additionally, inflammatory mediators might underlie this relationship, as periodontitis can influence the blood levels of inflammatory markers, such as interleukin-6, C-reactive protein, high-sensitivity C-reactive protein, salivary metalloproteinase-8 and -9, and salivary myeloperoxidase [6–9], which is also a possible molecular mediator [10].
Over the past 20 years, an association between the number of teeth and chronic systemic diseases has gradually been made [11]. A few studies have shown that tooth loss may be associated with an increased risk of CHD events. Caries and periodontal disease are the main causes of tooth loss, and the strength of the relationship between the loss of attachment and the loss of teeth was reported in 1997 [12]. Missing teeth may be a broad independent indicator of an accumulation of oral inflammation. As shown in a recent study, patients with 1 to 9 teeth had C-reactive protein levels that were the highest of all inflammatory factors, and patients with 10 to 19 teeth had IgG levels that were the highest, suggesting that the number of remaining teeth can be used to predict cardiovascular disease in patients [13].
CHD affects millions of people in developed and developing countries and has caused serious social and economic stress on a global scale over the past several decades [14]. Periodontal disease and tooth loss are 2 common oral health measures. Owing to the high prevalence of periodontitis and CHD, studying the relationship between the 2 diseases is of great public health importance and can also have considerable economic implications. We searched the literature by using the PubMed, Embase, and Cochrane Library databases from inception to April 2020. We found there are 4 systematic reviews and 9 meta-analyses on the association of periodontitis or the number of teeth with CHD [15–27]. There are 11 studies on the association of periodontitis with CHD and 3 studies on the association of number of teeth with CHD. All 3 studies on the association of number of teeth with CHD showed that tooth loss is associated with a significant risk of cardiovascular disease [25–27]. However, periodontitis has been identified as a risk factor for CHD in 9 of 11 studies on the relationship between CHD and periodontal disease [15–22,25]. Two studies did not have enough evidence to support this view [23,24]. Therefore, the relationship between periodontitis and cardiovascular disease risk remains controversial. To update our understanding of the relationship between periodontitis and CHD and to study the relationship between CHD and tooth loss, this systematic review and meta-analysis was conducted by gathering information from cohort studies.
Material and Methods
Search Strategy
This systematic evaluation was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We searched the literature by using the PubMed, Embase and Cochrane Library databases from inception to April of 2020. The publication language was limited to English. Our search strategy in PubMed was as follows: “Coronary Disease” [MeSH] and “Periodontitis” [MeSH]. The keywords included the following: (periodontal disease [title/abstract] or periodontitis [title/abstract] or periodontal pocket [title/abstract] and (coronary diseases [title/abstract] or coronary heart disease [title/abstract] or coronary aneurysm [title/abstract] or coronary artery disease [title/abstract] or coronary occlusion [title/abstract] or coronary stenosis [title/abstract] or coronary restenosis [title/abstract] or coronary vasospasm [title/abstract] or coronary thrombosis [title/abstract] or coronary-subclavian steal syndrome [title/abstract]).
Eligibility Criteria
To be eligible for inclusion, the studies had to meet the following criteria: (1) participants in the research population were without a history of CHD at the start of study; (2) exposure variables were with or without periodontitis or the baseline number of teeth; (3) healthy population and 25 to 32 teeth in a control group; (4) fatality and incidence of CHD as a result of exposure, both of which were included as the risk of CHD; (5) prospective or cohort study that provided the hazard ratio (HR) or relative risk (RR) and its 95% confidence interval (CI) or provided data that could be used to calculate those variables.
The exclusion criteria included the following: (1) teeth lost during follow-up (incidence of tooth loss); (2) self-reported CHD, with subjective symptoms such as angina; (3) repeated reports, poor-quality study, or unavailable information.
In our analysis, the primary endpoint was the incidence of CHD or fatal CHD. The International Classification of Disease and Related Health Problems (ICD) guidelines were used to classify the cause of mortality or events of CHD. CHD events included the incidence of fatal and symptomatic nonfatal myocardial infarction (MI) and sudden death without plausible causes other than CHD.
Quality Assessment
The studies were evaluated based on the following: representativeness of the exposed cohort, ascertainment of exposure, selection of the non-exposed cohort, demonstration that the outcome of interest was not present at the start of the study, comparability of cohorts on the basis of the design or analysis, assessment of outcome, follow-up sufficiently long to observe outcomes, and the adequacy of the follow-up of cohorts. The quality scores of the selected articles were evaluated by 2 double-blind researchers, based on the Newcastle-Ottawa scale (NOS), which has a total score of 9 points; lower scores corresponded to the lower quality of the article (Table 1).
Table 1.
Newcastle-Ottawa scale (NOS) evaluation results.
| Reference | Exposed representation | Ascertainment of exposure | Selection of the non-exposed | Outcome was not present at start of study | Comparability of chorts | Assessment of outcome | Sufficient follow-up time | Adequacy of follow up of cohorts | Total |
|---|---|---|---|---|---|---|---|---|---|
| DeStefano et al, 1993 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Joshipura et al, 1996 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Morrison et al, 1999 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Hujoel et al, 2000 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Wu et al, 2000 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Howell et al, 2001 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 7 |
| Hung et al, 2004 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Mucci et al, 2009 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 7 |
| Holmlund et al, 2010 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Noguchi et al, 2014 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| Liljestrand et al, 2015 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 |
| Yu et al, 2015 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
The quality assessment values ranged from 0 to 9 stars. Each item scored 1 point, except for 1 item (comparability of cohorts), which could be given a maximum score of 2 points.
Data Extraction
Two researchers independently selected publications that were in accordance with the inclusion and exclusion criteria. When disagreements arose, a third researcher reviewed the study after additional discussion. The following information was extracted: first author, publication year, sample size, follow-up year, baseline number of teeth, assessment of the CHD, HR, or RR, 95% CI, and exposure to risk factors.
Statistical Methods and Data Synthesis
STATA (version 12.0, StataCorp, USA) was used for the systematic review. To account for clinical diagnoses, the effects of tooth number on the risk of CHD were divided into 4 sets of criteria to calculate the common risk. This reference set was chosen to include as much of the dental data as possible: 32–25, 24–17, 16–11, and ≤10 teeth. Among those groups, the RR for no cases of periodontitis and the group with 32 to 25 teeth was 1. In the literature regarding the appropriate endpoint events, the unadjusted RRs were removed, and the results of different adjusted factors were assessed. The adjustment factors of the selected literature were divided mainly into 2 levels: (1) demographics and socioeconomic status, including age, sex, race, poverty index, marital status, and education, and (2) cardiovascular risk, including smoking, cigarettes, blood pressure, diabetes, and physical activity, and specific factors including smoking and hypertension. Standardized numbers of teeth and periodontal conditions were specified in the statistical software as subgroups with the fixed inverse-variance model. The Begg rank correlation test was used to estimate publication bias. Heterogeneity was evaluated by the I2 index and Cochrane Q statistics, where I2 ≥50% suggested that there was heterogeneity. A fixed-effects model was used if I2 <50%.
Results
Study Selection Process
A total of 877 studies were identified in the databases as potentially relevant articles, according to the search strategy. Using the inclusion and exclusion criteria, this meta-analysis included 11 articles in which periodontitis or the number of teeth was an exposure variable and CHD was an outcome variable. However, there were 5 articles in which patients with preexisting CHD were excluded in the study population. The flow diagram of the study selection process is shown in Figure 1.
Figure 1.
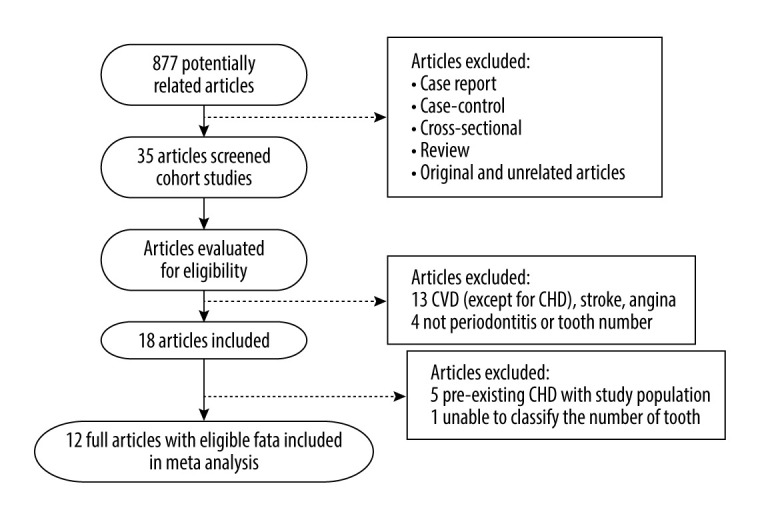
The flowchart of publication screening.
Baseline Characteristics of Included Studies
The characteristics of all articles are summarized in Table 2, which displays the main findings of the included studies. A total of 4 studies used number of teeth as their exposure variable, and 10 studies used periodontal condition as their exposure variable; the data on number of the teeth in 1 article could not be merged with the overall subgroups [28]. Joshipura et al, Howell et al, and Noguchi et al did not study women in the community, Yu et al studied only women, and Hung et al analyzed men and women separately. The study cohorts covered 20 to 89 years and met all the inclusion criteria. We included 2 types of exposure variables, and after removing the article that did not reveal a standardized tooth number, a total of more than 200 000 study participants were included in our study. The included prospective studies were conducted in 4 countries: America, Sweden, Canada, and Japan. Table 3 and Table 4 separately show the extracted information needed for the analysis, including the subgroups based on periodontal condition and number of teeth, HR or RR, 95% CI, and the adjusted factors.
Table 2.
Study characteristics.
| Reference | Sample size | Age range | Control group | Study area | Follow-up year | Periodontal condition/teeth number at baseline | CHD assessment |
|---|---|---|---|---|---|---|---|
| DeStefano et al, 1993 | 20749 | 25–74 | No periodontitis | USA | 14 | Periodontitis gingivitis edentulous |
CHD mortality and admission to hospital |
| Joshipura et al, 1996 | 44119 | 40–75male | No periodontitis 32-25 |
USA | 6 | Periodontitis 32-25; 24-17 16-11; 10-0 |
Incidence of fatal and non-fatal MI and sudden death |
| Morrison et al, 1999 | 16090 | 35–84 | No periodontitis | Canada | 23 | Periodontitis gingivitis edentulous |
Risk of fatal CHD |
| Hujoel et al, 2000 | 8032 | 25–74 | No periodontitis 32-25 |
USA | 6 | Periodontitis gingivitis 32-25; 24-17; 16-11; 10-4 |
Fatalities, hospitalization of CHD and revascularization |
| Wu et al, 2000 | 9962 | 25–54 | No periodontitis | USA | 21 | Periodontitis gingivitis edentulous |
Hospital records for non-fatal events and death certificates for fatal events |
| Howell et al, 2001 | 22037 | 40–84male | No periodontitis | USA | 12.3 | Periodontitis | Nonfatal MI |
| Hung et al, 2004 | 100381 | 40–75 male 30–55 female |
32-25 | USA | 12 | 32-25; 24-17; 16-11; 10-0 |
Symptomatic nonfatal MI, fatal CHD, or sudden death |
| Mucci et al, 2009 | 15273 | >35 | No periodontitis | Sweden | 35 | Periodontitis | Coronary heart disease (ICD-9 codes 410–414) |
| Holmlund et al, 2010 | 7674 | 20–89 | No periodontitis >25 |
Sweden | 12 | Periodontitis >25; 25-20; 19-15; 14-10; <10 |
ICD mortality (ICD-8, ICD-9, and ICD-10) |
| Noguchi et al, 2014 | 3081 | 36–59 male | No periodontitis | Japan | 5 | Periodontitis | MI |
| Liljestrand et al, 2015 | 8446 | 25–74 | 0–1 | Finland | 13 | 0–1; 2–4 5–8; 9–31; 32 |
A history of myocardial infarction, revascularizations, or percutaneous transluminal coronary angioplasty |
| Yu et al, 2015 | 39863 | ≥45 female | No periodontitis | USA | 15.7 | Periodontitis | MI |
The sample size includes the original population, not excluding loss. CHD – coronary heart disease; MI – myocardial infarction; ICD – International Classification of Disease.
Table 3.
Hazard ratio (HR) or risk ratio (RR) of included studies sorted by adjustment factors in meta-analysis with subgroup as periodontal condition.
| Reference | Subgroup | HR/RR | CI | Adjustment |
|---|---|---|---|---|
| DeStefano et al, 1993 | Gingivitis | 1.05 | 0.88–1.26 | Age, sex, race, education, poverty, alcohol, systolic blood pressure, marital status, BMI, smoking, physical activity, and cholesterol |
| PD | 1.25 | 1.06–1.48 | ||
| Edentulous | 1.23 | 1.05–1.44 | ||
| Joshipura et al, 1996 | PD | 1.04 | 0.97–1.25 | Age and smoking |
| 1.04 | 0.86–1.25 | Multivariate# | ||
| Morrison et al, 1999 | Mild gingivitis | 1.45 | 0.85–2.48 | Age and sex |
| Severe gingivitis | 1.82 | 1.09–3.06 | ||
| PD | 1.31 | 0.78–2.19 | ||
| Edentulous | 1.89 | 1.18–3.04 | Serum total cholesterol, diabetes status, smoking status, province of residence, hypertensive status, age and sex | |
| Mild gingivitis | 1.54 | 0.89–2.67 | ||
| Severe gingivitis | 2.15 | 1.25–3.72 | ||
| PD | 1.37 | 0.8–2.35 | ||
| Edentulous | 1.9 | 1.17–3.1 | ||
| Hujoel et al, 2000 | Gingivitis | 0.99 | 0.77–1.28 | Age, age squared, sex, race, education, poverty Index, marital status, interaction term for marital status and sex |
| 1.02 | 0.88–1.18 | |||
| PD | 1.28 | 1.02–1.61 | ||
| 1.24 | 1.08–1.43 | |||
| Gingivitis | 1.17 | 0.84–1.61 | Multivariate$ | |
| 1.05 | 0.88–1.26 | |||
| PD | 1.20 | 0.9–1.61 | ||
| 1.14 | 0.96–1.36 | |||
| Wu et al, 2000 | Gingivitis | 1.03 | 0.87–1.21 | several well-established CVD risk factors and demographic variables |
| PD | 1.14 | 0.98–1.34 | ||
| Edentulous | 1.13 | 0.98–1.32 | ||
| Howell et al, 2001 | PD | 1.01 | 0.87–1.17 | Age, aspirin, cigarette, smoking, alcohol, history of hypertension, BMI, diabetes, physical activity, history of MI and angina* |
| Mucci et al, 2009 | PD | 1.4 | 1.1–1.6 | Age, sex, education, smoking, diabetes, number of siblings, hypertension, and BMI |
| Holmlund et al, 2010 | Moderate PD | 0.73 | 0.26–2.01 | Age, gender, smoking |
| severe PD | 0.78 | 0.27–2.21 | ||
| Noguchi et al, 2014 | PD | 2.51 | 0.95–6.62 | Age |
| 2.26 | 0.84–6.02 | Age, smoking, diabetes, hypertension and BMI | ||
| Yu et al, 2015 | PD | 1.34 | 1.12–1.62 | Age |
| 1.35 | 1.05–1.73 | Multivariate& |
The multivariate model includes age (continuous); body mass index (BMI) (5 categories); exercise (5 categories); smoking habits (current smoker [number of cigarettes smoked: 1–14, 15–24, 25+], former smoker, or never smoked); alcohol consumption (7 categories); family history of myocardial infarction (MI) before 60 years of age; vitamin E (5 categories).
Multivariate model includes demographics and socioeconomic status: age, sex, race, poverty index, and cardiovascular risk factors (smoking, cigarettes, blood pressure, cholesterol, diabetes, height, physical activity).
Age (years), aspirin and beta-carotene treatment assignment, cigarette smoking, alcohol use, history of hypertension (systolic blood pressure ≥160 mmHg, diastolic blood pressure ≥95 mmHg or history of treatment for high blood pressure), BMI, reported history of diabetes, physical activity (reported vigorous exercise once per week or more), parental history of myocardial infarction (MI) and history of angina.
Multivariate model includes age, race/ethnicity, BMI, education, smoking, diabetes, hypertension, family history of MI, physical activities, and C-reactive protein.
BMI – body mass index; HR/RR – hazard ratio/relative risk; CI – confidence interval; MI – myocardial infarction.
Table 4.
Hazard ratio (HR) or risk ratio (RR) of included studies sorted by adjustment factors in meta-analysis with subgroup as number of teeth.
| Reference | Subgroup | Tooth count | HR/RR | CI | Adjustment |
|---|---|---|---|---|---|
| Joshipura et al, 1996 | 24-17 | Remain tooth | 1.08 | 0.88–1.33 | Age, smoking |
| 16-11 | 1.12 | 0.76–1.64 | |||
| 10-06 | 1.4 | 1.04–1.87 | |||
|
| |||||
| 24-17 | 1.04 | 0.85–1.28 | Age; BMI; exercise; smoking habits; alcohol consumption; family history of MI before 60 years of age; VEc | ||
| 16-11 | 1.06 | 0.72–1.56 | |||
| 10-0 | 1.32 | 0.98–1.77 | |||
|
| |||||
| 24-17 | 1.03 | 0.83–1.27 | Multivariate+dietd | ||
| 16-11 | 1.04 | 0.71–1.54 | |||
| 10-0 | 1.29 | 0.96–1.73 | |||
|
| |||||
| Hujoel et al, 2000 | 24-17 | Remain tooth | 0.84 | 0.64–1.09 | Multivariate$ |
| 16-11 | 0.75 | 0.51–1.10 | |||
| 10-4 | 0.88 | 0.62–1.25 | |||
|
| |||||
| Hung et al, 2004 | 24-17 (man)a | Remain tooth | 1.15 | 1.00–1.32 | Age, smoking |
| 16-11 | 1.41 | 1.11–1.80 | |||
| 10-0 | 1.49 | 1.22–1.83 | |||
| 24-17 (woman)a | 1.28 | 1.03–1.60 | |||
| 16-11 | 1.6 | 1.15–2.22 | |||
| 10-0 | 2.13 | 1.70–2.65 | |||
|
| |||||
| 24-17 (man)a | 1.1 | 0.95–1.26 | Age, smoking, alcohol consumption, BMI, physical activity, family history of MI, multivitamin supplement, VE, hypertension, diabetes, hormone use, hypercholesterolemia in both cohorts and professions for men only, menopausal status | ||
| 16-11 | 1.35 | 1.06–1.72 | |||
| 10-0 | 1.36 | 1.11–1.67 | |||
| 24-17 (man)b | 1.26 | 1.01–1.57 | |||
| 16-11 | 1.19 | 0.79–1.80 | |||
| 10-0 | 1.79 | 1.34–2.40 | |||
| 24-17 (woman)a | 1.14 | 0.92–1.42 | |||
| 16-11 | 1.34 | 0.97–1.87 | |||
| 10-0 | 1.64 | 1.31–2.05 | |||
| 24-17 (woman)b | 1.02 | 0.66–1.55 | |||
| 16-11 | 1.07 | 0.56–2.05 | |||
| 10-0 | 1.65 | 1.11–2.46 | |||
|
| |||||
| Holmlund et al, 2010 | 25-20 | Remain tooth | 1.94 | 1.17–3.21 | Age, gender, and smoking |
| 19-15 | 3.45 | 2.04–5.84 | |||
| 14-10 | 3.3 | 1.82–5.99 | |||
| <10 | 7.33 | 4.11–13.07 | |||
|
| |||||
| Liljestrand et al, 2015 | 2–4 | Tooth loss | 1.22 | 0.81–1.85 | Age, sex, smoking, geographic variable, systolic blood pressure, blood pressure treatment, cholesterol, HDL cholesterol, education, existing diabetes |
| 5–8 | 1.62 | 1.08–2.43 | |||
| 9–31 | 1.99 | 1.37–2.89 | |||
| 32 | 1.65 | 1.09–2.50 | |||
Coronary heart disease (CHD) incidence: symptomatic nonfatal myocardial infarction (MI), fatal CHD, or sudden death.
CHD mortality.
Age (continuous); body mass index (5 categories); exercise (5 categories); smoking habits (current smoker [number of cigarettes smoked: 1–14, 15–24, 25+], former smoker, or never smoked); alcohol consumption (7 categories); family history of myocardial infarction before 60 years of age; vitamin E (5 categories).
Dietary variables include dietary fiber and carrots.
Indicates demographics and socioeconomic status, including age, sex, race, poverty index, and also cardiovascular risk factors (smoking, cigarettes, blood pressure, cholesterol, diabetes, height, physical activity).
HDL – high-density lipoprotein; VE – vitamin E.
Newcastle-Ottawa Scale
The quality score of the selected articles was evaluated by 2 double-blind researchers based on the NOS, which has a total possible score of 9 points; lower scores indicated a lower quality of the article. All of the included articles were assessed using the modified version of the NOS for cohort studies (Table 1) [2,28–37].
Meta-Analysis
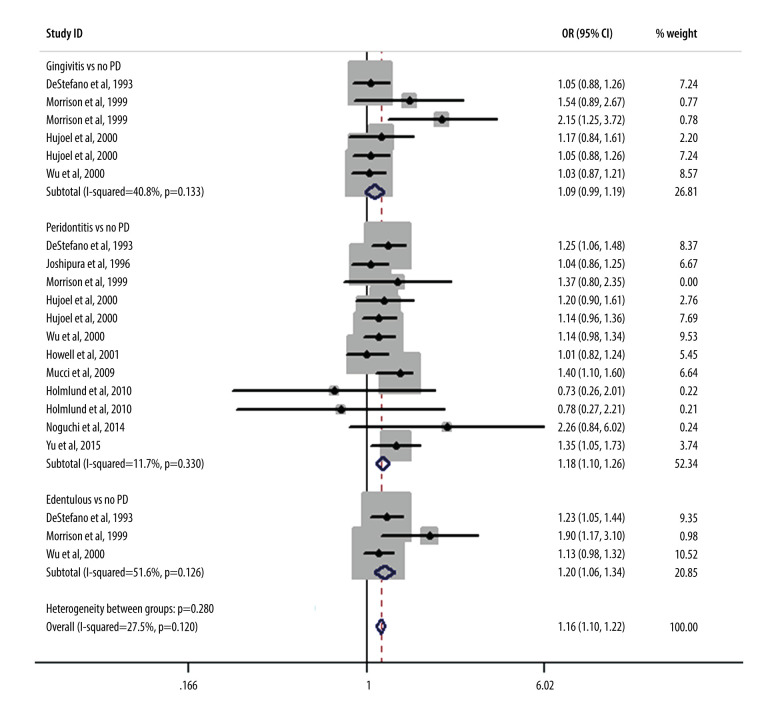
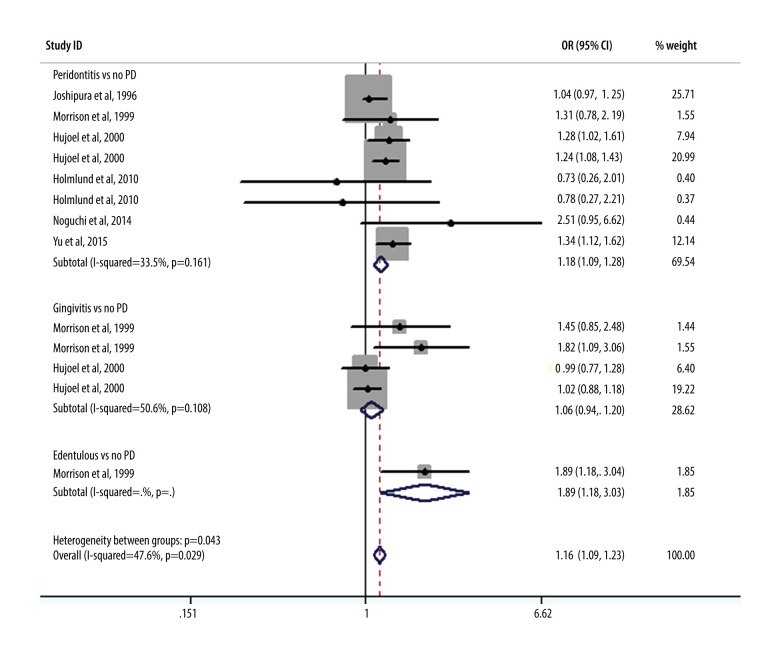
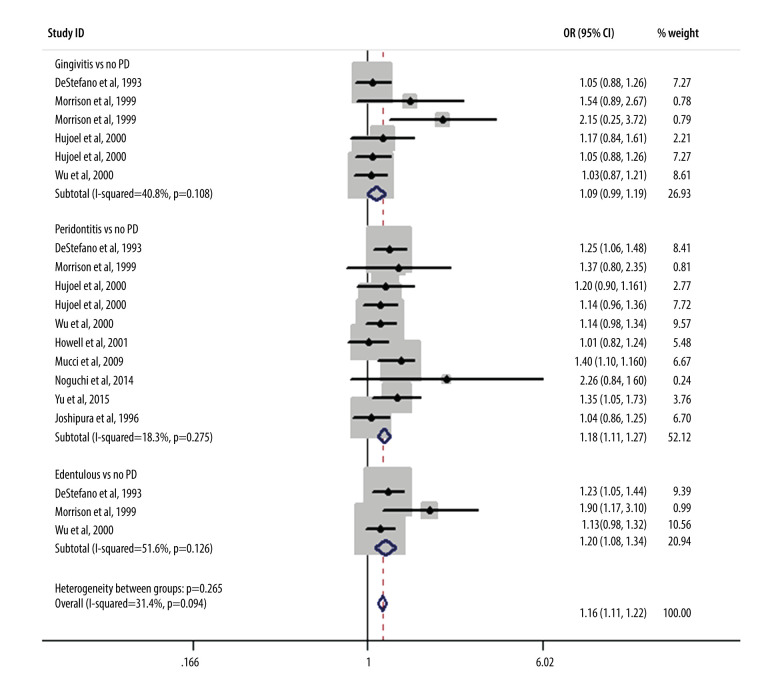
As shown in Figure 2, we performed a meta-analysis of the pooled RRs using the data from 10 included studies to assess the effect of periodontal condition on CHD. The results showed that after merging the confounding factors, the risk of CHD in patients who had periodontitis was 1.18 times (95% CI, 1.10–1.26) that of patients who did not have periodontitis. Only 1 study had an RR value markedly less than 1, showing no association between periodontitis and CHD. Further statistical tests revealed that the risk of CHD in patients with gingivitis also weakly increased over time (RR, 1.09; 95% CI, 0.99–1.19) compared with that in patients without periodontitis. Interestingly, among the edentulous population, there were also marked differences in the ratios of CHD risk (RR, 1.20; 95% CI, 1.08–1.34). Figure 3 shows the results obtained after adjustments were made for demographic and socioeconomic status; there were also significant positive correlations between CHD risk and gingivitis (RR, 1.06; 95% CI, 0.94–1.20), periodontitis (RR, 1.18; 95% CI, 1.09–1.28), and patients who were edentulous (RR, 1.89; 95% CI, 1.18–3.03). Figure 4 refers to smoking and hypertension factors; the RR value was not obviously altered (1.18; 95% CI, 1.11–1.27) with an I2 of 18.3%. Also, the overall heterogeneity assessment of multivariate confounding factors was 27.5%, although in Figure 3 it is 47.6%.
Figure 2.
Forest plot of comparison of gingivitis vs control (no periodontitis), periodontitis vs control, edentulous vs control. The outcome is coronary heart disease after the confounding factors were merged.
Figure 3.
Meta-analysis results of the relationship between periodontal condition and the risk of coronary heart disease adjusted by demographics and socioeconomic status.
Figure 4.
Meta-analysis results of the relationship between periodontal condition and the risk of coronary heart disease adjusted by smoking and hypertension.
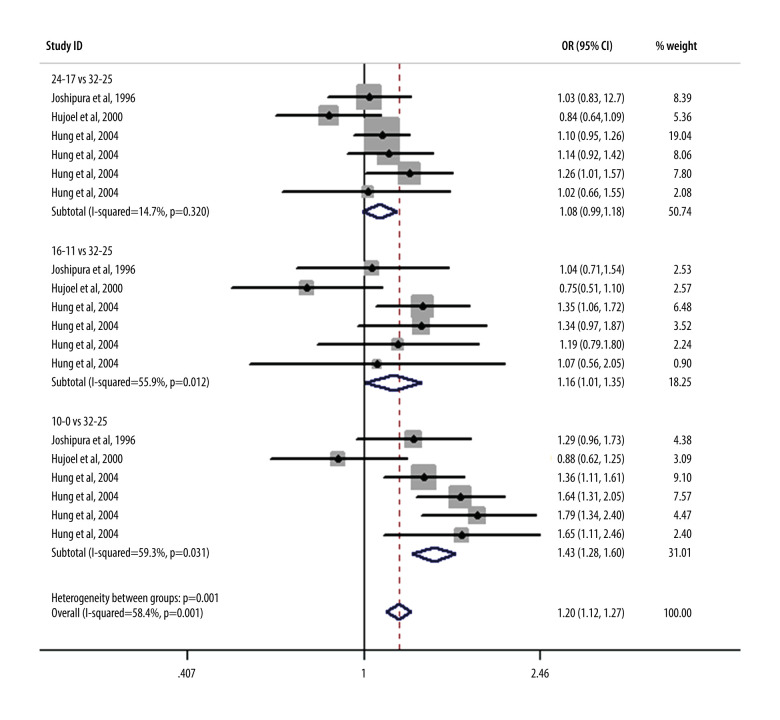
The single most striking observation from the data analysis was the result of the correlation analysis of the association between number of teeth and CHD risk, which is summarized in Figure 5. As shown, after adjusting for confounding factors, there was a significant association between the number of teeth and CHD risk, in which a lower number of teeth correlated with a greater CHD risk: 24–17 teeth vs 32–25 teeth (RR, 1.12; 95% CI, 1.05–1.19); 16–11 teeth vs 32–25 teeth (RR, 1.28; 95% CI, 1.15–1.42); and ≤10 teeth vs 32–25 teeth (RR, 1.55; 95% CI, 1.43–1.69). After we found the high heterogeneity presented in Figure 5 and no single relevant CHD adjustment factor, including age, sex, and smoking, we deleted the Holmlund study. The adjustment factors in Figure 6 are smoking and hypertension, which shows the risk of CHD after sensitivity analysis. The I2 value, which was lower than the original data, was 59.3% in the ≤10 teeth group. Figure 6 shows the relationship between the number of teeth and the risk of coronary heart disease after sensitivity analysis: 24–17 teeth vs 32–25 teeth (RR, 1.08; 95% CI, 0.99–1.18); 16–11 teeth vs 32–25 teeth (RR, 1.16; 95% CI, 1.01–1.35); and ≤10 teeth vs 32–25 teeth (RR, 1.43; 95% CI, 1.28–1.60).
Figure 5.
Meta-analysis results of relationship between the number of teeth and the risk of coronary heart disease after the confounding factors were merged.
Figure 6.
Meta-analysis results of the relationship between the number of teeth and the risk of coronary heart disease after sensitivity analysis.
Analysis of Publication Bias
The Begg plot in Figure 7 shows the estimated publication bias of 10 studies on periodontal disease. The plot shows basic graphic symmetry and no evidence of significant publication bias; however, the possibility of bias is not excluded (P>0.05).
Figure 7.
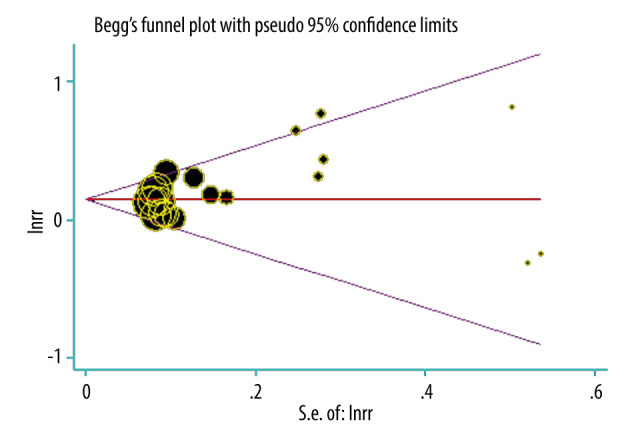
Publication bias in reference to periodontal disease.
Discussion
A number of systematic reviews and meta-analyses have indicated that the presence of periodontal disease could be associated with an increased risk of CHD. However, the relationship between periodontitis and cardiovascular disease risk remains controversial. For example, among the 11 articles we searched [15–25], 9 articles considered periodontitis as a risk factor for CHD [15–22,25], but 2 articles did not have enough evidence to support this view [23,24]. Researchers had gradually identified an association between the number of teeth and CHD. However, few studies reported the influence of the number of teeth on the risk of CHD. We need more epidemiological evidence to show that tooth loss is harmful for CHD. Additionally, these relevant systematic reviews and meta-analyses have significant differences in methodological conduct. One of the main issues was that these published systematic reviews and meta-analyses pooled data from studies with different designs, namely prospective cohort, retrospective cohort, case-control, and cross-sectional studies [39]. In the 13 systematic reviews/meta-analyses [15–27], only the report by Leng et al included prospective cohort studies [20]. To update our understanding of the relationship between periodontitis and CHD and to study the relationship between CHD and tooth loss, our study included prospective cohort studies only and analyzed 2 types of exposure variables: periodontitis and the baseline number of teeth.
In 2018, Cheng et al [27] published a study to identify and quantify the potential dose-response association between tooth loss, cardiovascular disease, and stroke risk. Their meta-analysis results suggested that tooth loss is independently associated with deleterious cardiovascular disease and incremental stroke risk. The results showed a statistically significant incremental association between tooth loss and cardiovascular disease, with a 3% incremental increase of cardiovascular disease risk per 2 lost teeth. Compared with their study, our present study was a prospective cohort study. Also, we developed a more detailed classification of the relationship between the number of teeth and CHD, with the number of teeth divided into 4 subsets: 32–25 teeth, 24–17 teeth, 16–11 teeth, and ≤10 teeth. We then calculated the risk of CHD for each of those individual sets.
The results of our study demonstrated a statistically significant relationship between periodontal disease and the risk of CHD (RR, 1.18; 95% CI, 1.10–1.26). Furthermore, we identified a 1.55 times increased risk (95% CI, 1.43–1.69) for CHD in patients with fewer than 10 teeth. The RRs for patients with 24 to 17 teeth and 16 to 11 teeth were 1.12 and 1.28, respectively. The risk of CHD increased to 1.89 for edentulous patients. These data show the positive association of teeth number with CHD risk, with a lower baseline number of teeth indicating a higher risk of CHD. Based on the result of the present meta-analysis, the prevalence and incidence of CHD were significantly increased in patients with periodontitis, and this conclusion is consistent with that of a previous meta-analysis [40]. Because of the complexity and necessity of accurate cardiovascular risk prediction, the 2019 guidelines for chronic coronary syndromes from the European Society of Cardiology (ESC) provided more detailed and critical elements based on the findings of diagnostic cardiovascular tests [41]. This guideline specified the definition of levels of risk. In addition to the number of teeth, cardiovascular disease risk is also linked to the occurrence of ST segment depression, low exercise capacity, and abnormal blood pressure response.
The eligibility criteria for studies we included in our analysis were strict. The cohort study by Bahekar et al included patients who already had coronary artery disease. Another prospective cohort meta-analysis had a similar association [42]. Three trials were excluded from our study for CHD because the index of periodontal disease was different [43–45]. Study results have demonstrated a meaningful relationship between periodontitis and the existence of various types of cardiovascular disease; for example, Shi et al assessed the strength of the association between MI and periodontitis in case-controlled studies [46]. Indeed, a member of the transforming growth factor-β signaling pathway may be a shared genetic risk factor of cardiovascular disease and periodontitis, and proinflammatory genetic factors may also explain some of our present findings [47]. The exclusion of self-reported CHD and subjective symptoms like angina from our study were to minimize self-report bias and improve the accuracy of our results, because these diagnoses had not been confirmed by a clinician.
The relationship between the risk of tooth loss and CHD is explained by the association of oral infection and concomitant inflammation with periodontal disease, whereby severe periodontal disease causes tooth loss or even an edentulous jaw [48]. One study on advanced periodontitis in adults found the elimination of advanced periodontitis via full-mouth teeth extraction can reduce systemic inflammation and thrombotic cardiovascular disease risk [49]. Another study found that local synthesis of inflammatory cytokines causes periodontal tissue breakdown, resulting in loss of teeth, which leads to the penetration of oral pathogenic bacteria and bacterial products into the bloodstream, ultimately causing the increase in cardiovascular events [11]. The findings of Vedin et al illustrated that characteristic biomarkers (high-sensitivity C-reactive protein, interleukin-6, lipoprotein-associated phospholipase A2, and N-terminal pro-B-type natriuretic peptide) were highly expressed in populations with the highest tooth loss, using multivariate analysis [50].
Regarding unhealthy periodontal conditions, there existed the similar bidirectional logical relationship in teeth number on account of periodontitis and systemic diseases. Results of a 40-year cohort study showed that adults with diabetes have lost twice as many teeth as those without diabetes [51–53]. In addition, other diseases give rise to poor oral health conditions, such as salivary gland disease, radiotherapy complications, and AIDS. However, oral bacteria and metabolites cause other unexplained infectious and non-infectious diseases (sepsis, osteomyelitis, arthritis). At the same time, almost all tooth loss is caused by the bacterial population correlated to periodontitis or caries [54–56]. It has also been reported that missing teeth are owing to lifestyle factors such as unhealthy nutrition or smoking, oral care awareness, and educational and socioeconomic background [57].
Missing teeth can directly or indirectly affect dietary habits, thereby changing nutrition intake. It is known that the decrease in micronutrient intake from fruits and vegetables following tooth loss can explain the association between the number of teeth and CHD risk [58]. Batty et al showed the relationship between cigarette smoking or drinking and poor oral health, such as tooth loss, and its relationship to CHD, providing another indirect explanation [38]. Although the association between CHD and periodontal disease is well established, health care professionals also need to recognize the risk of tooth loss and its effect on quality of life among people with CHD.
Advantages of the present research study, in contrast to other studies, are the strict inclusion criteria for the population, periodontitis diagnostic indicators, recording of number of teeth, and update of the relevant data of cohort studies. There are also several limitations. The grouping of individuals based on the number of teeth was done differently in the various articles, and the present study ignored the differences in individual teeth, which can impact the accuracy of the result through high heterogeneity. Moreover, the number of teeth does not fully represent oral inflammation, even though most cases of tooth loss are caused by periodontitis; other diseases can also cause tooth loss. Further study should explore the interaction between periodontitis and other cardiovascular events, such as ischemic heart disease or stroke.
Conclusions
In this present systematic review of cohort studies, the association between periodontitis and CHD was found to be meaningful, as was the preliminary exploration of the effect of a reduced number of teeth on CHD. Although estimating cardiovascular risk is still complicated in clinical work, the number of teeth is considered a risk factor for cardiovascular disease, however, not an independent one. Verifying the explicit relationship between number of teeth and CHD requires the further study of biological mechanisms.
Footnotes
Conflicts of Interest
None.
Financial support: Departmental sources
References
- 1.Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Inter J Health Sci. 2017;11:72–80. [PMC free article] [PubMed] [Google Scholar]
- 2.DeStefano F, Anda RF, Kahn HS, et al. Dental disease and risk of coronary heart disease and mortality. Br Med J. 1993;306:688–91. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elter JR, Champagne CM, Offenbacher S, et al. Relationship of periodontal disease and tooth loss to prevalence of coronary heart disease. J Periodontol. 2004;75:782–90. doi: 10.1902/jop.2004.75.6.782. [DOI] [PubMed] [Google Scholar]
- 4.Lopez R, Oyarzun M, Naranjo C, et al. Coronary heart disease and periodontitis – a case control study in Chilean adults. J Clin Periodontol. 2002;29:468–73. doi: 10.1034/j.1600-051x.2002.290513.x. [DOI] [PubMed] [Google Scholar]
- 5.Holmlund A, Lampa E, Lind L. Oral health and cardiovascular disease risk in a cohort of periodontitis patients. Atherosclerosis. 2017;262:101–6. doi: 10.1016/j.atherosclerosis.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Anitha V, Nair S, Shivakumar V, et al. Estimation of high-sensitivity C-reactive protein in patients with periodontal disease and without coronary artery disease. Indian J Dent Res. 2015;26:500–3. doi: 10.4103/0970-9290.172048. [DOI] [PubMed] [Google Scholar]
- 7.Bing XJ, Sun XJ, Shen GH, et al. [Levels of IL-8, IL-10 in patients with chronic periodontitis and coronary heart disease]. Shanghai Kou Qiang Yi Xue. 2015;24:598–601. [in Chinese] [PubMed] [Google Scholar]
- 8.Kumar KR, Ranganath V, Naik R, et al. Assessment of high-sensitivity C-reactive protein and lipid levels in healthy adults and patients with coronary artery disease, with and without periodontitis-a cross-sectional study. J Periodontal Res. 2014;49:836–44. doi: 10.1111/jre.12172. [DOI] [PubMed] [Google Scholar]
- 9.Rathnayake N, Gustafsson A, Norhammar A, et al. Salivary matrix metalloproteinase-8 and -9 and myeloperoxidase in relation to coronary heart and periodontal diseases: A subgroup report from the PAROKRANK study (periodontitis and its relation to coronary artery disease) PLoS One. 2015;10:e0126370. doi: 10.1371/journal.pone.0126370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liljestrand JM, Paju S, Buhlin K, et al. Lipopolysaccharide, a possible molecular mediator between periodontitis and coronary artery disease. J Clin Periodontol. 2017;44:784–92. doi: 10.1111/jcpe.12751. [DOI] [PubMed] [Google Scholar]
- 11.Oluwagbemigun K, Dietrich T, Pischon N, et al. Association between number of teeth and chronic systemic diseases: A cohort study followed for 13 years. PLoS One. 2015;10:e0123879. doi: 10.1371/journal.pone.0123879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck JD, Sharp T, Koch GG, et al. A 5-year study of attachment loss and tooth loss in community-dwelling older adults. J Periodontal Res. 1997;32:516–23. doi: 10.1111/j.1600-0765.1997.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 13.Aoyama N, Suzuki JI, Kobayashi N, et al. Associations among tooth loss, systemic inflammation and antibody titers to periodontal pathogens in Japanese patients with cardiovascular disease. J Periodontal Res. 2018;53:117–22. doi: 10.1111/jre.12494. [DOI] [PubMed] [Google Scholar]
- 14.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: Concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50(22):2128–32. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 15.Blaizot A, Vergnes JN, Nuwwareh S, et al. Periodontal diseases and cardiovascular events: Meta-analysis of observational studies. Int Dent J. 2009;59(4):197–209. [PubMed] [Google Scholar]
- 16.Cronin A. Periodontal disease is a risk marker for coronary heart disease? Evid Based Dent. 2009;10(1):22. doi: 10.1038/sj.ebd.6400634. [DOI] [PubMed] [Google Scholar]
- 17.Hujoel PP. Does chronic periodontitis cause coronary heart disease? A review of the literature. J Am Dent Assoc. 2002;133(Suppl):31s–36s. doi: 10.14219/jada.archive.2002.0377. [DOI] [PubMed] [Google Scholar]
- 18.Humphrey LL, Fu R, Buckley DI, et al. Periodontal disease and coronary heart disease incidence: A systematic review and meta-analysis. J Gen Intern Med. 2008;23(12):2079–86. doi: 10.1007/s11606-008-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janket SJ, Baird AE, Chuang SK, et al. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(5):559–69. doi: 10.1067/moe.2003.107. [DOI] [PubMed] [Google Scholar]
- 20.Leng WD, Zeng XT, Kwong JS, et al. Periodontal disease and risk of coronary heart disease: An updated meta-analysis of prospective cohort studies. Int J Cardiol. 2015;201:469–72. doi: 10.1016/j.ijcard.2015.07.087. [DOI] [PubMed] [Google Scholar]
- 21.Shah KK, Vishnu Priya V. Link between periodontal diseases and coronary artery diseases: A systemic review. Int J Pharm Sci Rev Res. 2015;33(1):154–56. [Google Scholar]
- 22.Xiao-Ping W, Wei L, Yu-Shan H. Correlation between chronic periodontitis and cardiovascular diseases: A systematic review. Heart. 2012;98:E93–94. [Google Scholar]
- 23.Khader YS, Albashaireh ZS, Alomari MA, et al. Periodontal diseases and the risk of coronary heart and cerebrovascular diseases: A meta-analysis. J Periodontol. 2004;75(8):1046–53. doi: 10.1902/jop.2004.75.8.1046. [DOI] [PubMed] [Google Scholar]
- 24.Madianos PN, Bobetsis GA, Kinane DF, et al. Is periodontitis associated with an increased risk of coronary heart disease and preterm and/or low birth weight births? J Clin Periodontol. 2002;29(3):22–36. doi: 10.1034/j.1600-051x.29.s3.2.x. [DOI] [PubMed] [Google Scholar]
- 25.Bahekar AA, Singh S, Saha S, et al. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: A meta-analysis. Am Heart J. 2007;154(5):830–37. doi: 10.1016/j.ahj.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 26.Joshipura KJ, Douglass CW, Willett WC, et al. Possible explanations for the tooth loss and cardiovascular disease relationship. Ann Periodontol. 1998;3(1):175–83. doi: 10.1902/annals.1998.3.1.175. [DOI] [PubMed] [Google Scholar]
- 27.Cheng F, Zhang M, Wang Q, et al. Tooth loss and risk of cardiovascular disease and stroke: A dose-response meta-analysis of prospective cohort studies. PLoS One. 2018;13(3):e0194563. doi: 10.1371/journal.pone.0194563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshipura KJ, Rimm EB, Douglass CW, et al. Poor oral health and coronary heart disease. J Dent Res. 1996;75:1631–36. doi: 10.1177/00220345960750090301. [DOI] [PubMed] [Google Scholar]
- 29.Morrison HI, Ellison LF, Taylor GW. Periodontal disease and risk of fatal coronary heart and cerebrovascular diseases. J Cardiovasc Risk. 1999;6:7–11. doi: 10.1177/204748739900600102. [DOI] [PubMed] [Google Scholar]
- 30.Hujoel PP, Drangsholt M, Spiekerman C, et al. Periodontal disease and coronary heart disease risk. JAMA. 2000;284:1406–10. doi: 10.1001/jama.284.11.1406. [DOI] [PubMed] [Google Scholar]
- 31.Wu T, Trevisan M, Genco R, et al. Periodontal disease as a risk factor for CVD, CHD, and stroke. Circulation. 2000;99:1109–25. [Google Scholar]
- 32.Howell TH, Ridker PM, Ajani UA, et al. Periodontal disease and risk of subsequent cardiovascular disease in U.S. male physicians. J Am Coll Cardio. 2001;37:445–50. doi: 10.1016/s0735-1097(00)01130-x. [DOI] [PubMed] [Google Scholar]
- 33.Hung HC, Joshipura KJ, Colditz G, et al. The association between tooth loss and coronary heart disease in men and women. J Public Health Dent. 2004;64:209–15. doi: 10.1111/j.1752-7325.2004.tb02755.x. [DOI] [PubMed] [Google Scholar]
- 34.Mucci LA, Hsieh CC, Williams PL, et al. Do genetic factors explain the association between poor oral health and cardiovascular disease? A prospective study among Swedish twins. Am J Epidemiol. 2009;170:615–21. doi: 10.1093/aje/kwp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmlund A, Holm G, Lind L. Number of teeth as a predictor of cardiovascular mortality in a cohort of 7,674 subjects followed for 12 years. J Periodontol. 2010;81:870–76. doi: 10.1902/jop.2010.090680. [DOI] [PubMed] [Google Scholar]
- 36.Noguchi S, Toyokawa S, Miyoshi Y, et al. Five-year follow-up study of the association between periodontal disease and myocardial infarction among Japanese male workers: MY Health Up Study. J Public Health. 2015;37(4):345–52. doi: 10.1093/pubmed/fdu076. [DOI] [PubMed] [Google Scholar]
- 37.Yu YH, Chasman DI, Buring JE, et al. Cardiovascular risks associated with incident and prevalent periodontal disease. J Clin Periodontol. 2015;42:21–28. doi: 10.1111/jcpe.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batty GD, Jung KJ, Mok Y, et al. Oral health and later coronary heart disease: Cohort study of one million people. Eur J Prev Cardiol. 2018;25(6) doi: 10.1177/2047487318759112. 2047487318759112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly JT, Avila-Ortiz G, Allareddy V, et al. The association between periodontitis and coronary heart disease: A quality assessment of systematic reviews. J Am Dent Assoc. 2013;144:371–79. doi: 10.14219/jada.archive.2013.0130. [DOI] [PubMed] [Google Scholar]
- 40.Bahekar AA, Singh S, Saha S, et al. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: A meta-analysis. Am Heart J. 2007;154:830–37. doi: 10.1016/j.ahj.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 41.Juhani K, William W, Antti S, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–77. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 42.Leng WD, Zeng XT, Kwong JS, et al. Periodontal disease and risk of coronary heart disease: An updated meta-analysis of prospective cohort studies. Int J Cardiol. 2015;201:469–72. doi: 10.1016/j.ijcard.2015.07.087. [DOI] [PubMed] [Google Scholar]
- 43.Dietrich T, Jimenez M, Krall Kaye EA, et al. Age-dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation. 2008;117:1668–74. doi: 10.1161/CIRCULATIONAHA.107.711507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuominen R, Reunanen A, Paunio M, et al. Oral health indicators poorly predict coronary heart disease deaths. J Dent Res. 2003;82:713–18. doi: 10.1177/154405910308200911. [DOI] [PubMed] [Google Scholar]
- 45.Reichert S, Schlitt A, Beschow V, et al. Use of floss/interdental brushes is associated with lower risk for new cardiovascular events among patients with coronary heart disease. J Periodontal Res. 2015;50:180–88. doi: 10.1111/jre.12191. [DOI] [PubMed] [Google Scholar]
- 46.Shi Q, Zhang B, Huo N, et al. Association between myocardial infarction and periodontitis: A meta-analysis of case-control studies. Front Physiol. 2016;7:519. doi: 10.3389/fphys.2016.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaefer AS, Bochenek G, Jochens A, et al. Genetic evidence for plasminogen as a shared genetic risk factor of coronary artery disease and periodontitis. Circ Cardiovasc Genet. 2015;8:159–67. doi: 10.1161/CIRCGENETICS.114.000554. [DOI] [PubMed] [Google Scholar]
- 48.Taguchi A, Sanada M, Suei Y, et al. Tooth loss is associated with an increased risk of hypertension in postmenopausal women. Hypertension. 2004;43:1297–300. doi: 10.1161/01.HYP.0000128335.45571.ce. [DOI] [PubMed] [Google Scholar]
- 49.Taylor BA, Tofler GH, Carey HM, et al. Full-mouth tooth extraction lowers systemic inflammatory and thrombotic markers of cardiovascular risk. J Dent Res. 2006;85:74–78. doi: 10.1177/154405910608500113. [DOI] [PubMed] [Google Scholar]
- 50.Vedin O, Hagstrom E, Ostlund O, et al. Associations between tooth loss and prognostic biomarkers and the risk for cardiovascular events in patients with stable coronary heart disease. Inter J Cardiol. 2017;245:271–76. doi: 10.1016/j.ijcard.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 51.Luo H, Pan W, Sloan F, et al. Peer reviewed: Forty-year trends in tooth loss among American adults with and without diabetes mellitus: An age-period-cohort analysis. Prev Chronic Dis. 2015;12:E211. doi: 10.5888/pcd12.150309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jimenez M, Hu FB, Marino M, et al. Type 2 diabetes mellitus and 20 year incidence of periodontitis and tooth loss. Diabetes Res Clin Pract. 2012;98(3):494–500. doi: 10.1016/j.diabres.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaur G, Holtfreter B, Rathmann WG, et al. Association between type 1 and type 2 diabetes with periodontal disease and tooth loss. J Clin Periodontol. 2012;36(9):765–74. doi: 10.1111/j.1600-051X.2009.01445.x. [DOI] [PubMed] [Google Scholar]
- 54.Chatrchaiwiwatana S, Ratanasiri A, Jaidee J, et al. Factors related to tooth loss due to dental caries among workers in an industrial estates in Thailand. J Med Assoc Thai. 2012;95(Suppl.11):S1–6. [PubMed] [Google Scholar]
- 55.Ravald N, Johansson CS. Tooth loss in periodontally treated patients. A long-term study of periodontal disease and root caries. J Clin Periodontol. 2015;39(1):73–79. doi: 10.1111/j.1600-051X.2011.01811.x. [DOI] [PubMed] [Google Scholar]
- 56.Schwendicke F, Schmietendorf E, Plaumann A, et al. Validation of multivariable models for predicting tooth loss in periodontitis patients. J Clin Periodontol. 2018;45(6):701–10. doi: 10.1111/jcpe.12900. [DOI] [PubMed] [Google Scholar]
- 57.Hassel AJ, Safaltin V, Grill S, et al. Risk factors for tooth loss in middle and older age after up to 10 years: An observational cohort study. Arch Oral Biol. 2018;86:7. doi: 10.1016/j.archoralbio.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 58.Ritchie CS, Joshipura K, Hung HC, et al. Nutrition as a mediator in the relation between oral and systemic disease: Associations between specific measures of adult oral health and nutrition outcomes. Crit Rev Oral Biol M. 2002;13:291–300. doi: 10.1177/154411130201300306. [DOI] [PubMed] [Google Scholar]