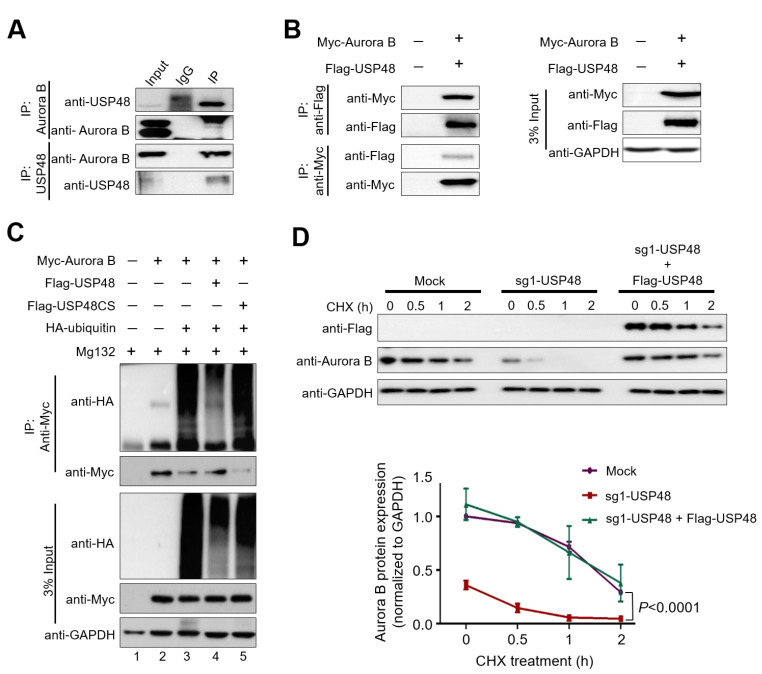
Figure 3.
USP48 interacts, deubiquitinates, and extends Aurora B protein half-life. (A) The endogenous interactions between the USP48 and Aurora B proteins were examined by immunoprecipitation (IP) experiments in HeLa cells. Cell lysates from HeLa cells were immunoprecipitated and immunoblotted with specific USP48 or Aurora B antibodies as indicated. (B) Exogenous IP experiments to demonstrate the interaction between Myc-Aurora B and Flag-USP48 were performed in HEK293 cells. Samples were immunoprecipitated and immunoblotted using either anti-Flag or anti-Myc antibodies as indicated. GAPDH was used as a loading control. (C) Myc-Aurora B and HA-ubiquitin were co-transfected along with either Flag-USP48 or Flag-USP48CS in HEK293 cells. The ubiquitination level of Aurora B protein was confirmed by performing IP with an anti-Myc antibody and immunoblotting with the anti-HA (ubiquitin) and anti-Myc antibody as indicated. (D) The cycloheximide assay was performed to demonstrate the half-life of endogenous Aurora B protein in cells transfected with mock control, sgRNA1 targeting USP48, and upon the reconstitution with Flag-USP48 in sgRNA1 targeted USP48 HeLa cells. Experiments were performed in triplicates and band intensities were estimated using ImageJ software with reference to the GAPDH control band and graphically represented. Two-way ANOVA followed by Tukey’s post hoc test was used and the p values are represented.