Figure 1.
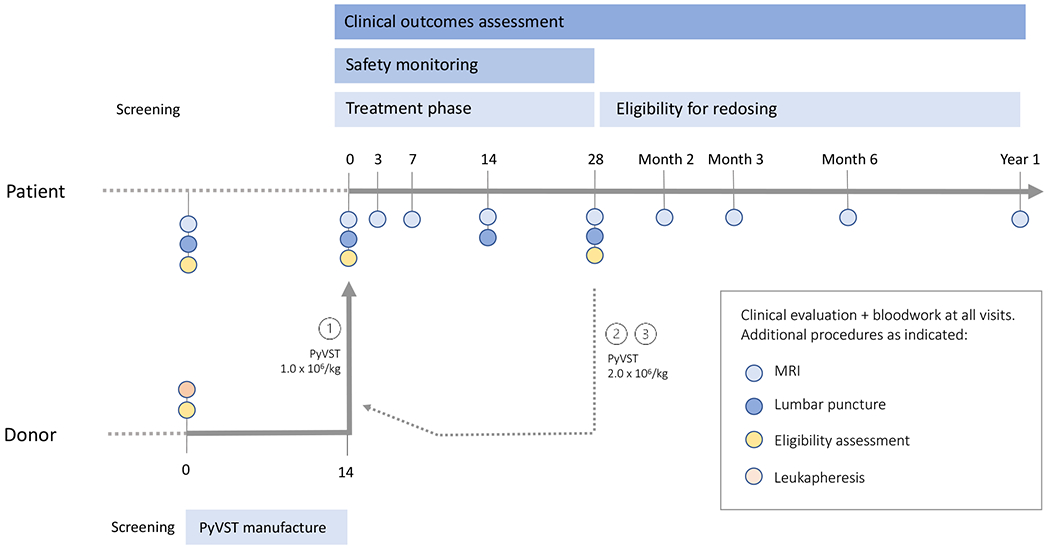
Trial schema.
Candidate patients and donors were screened for study eligibility contemporaneously. Selected donors underwent leukapheresis, and PyVST cultures were initiated either from cryopreserved peripheral blood mononuclear cells or freshly isolated cells. Final cell product was harvested at Day 14. Patients returned for baseline study visit and confirmation of eligibility criteria. First dose of PyVST was administered at 1x106 cells/kg followed by 28-day safety monitoring period with scheduled testing at Days 3, 7, 14 and 28. Patients were eligible for up to 2 additional doses of PyVST of 2x106 cells/kg, no less than 28 days from last infusion; each additional infusion was followed by 28-day safety monitoring as previously. Patients were followed for up to 1 year after last infusion with scheduled clinic visits and testing.
