Figure 3.
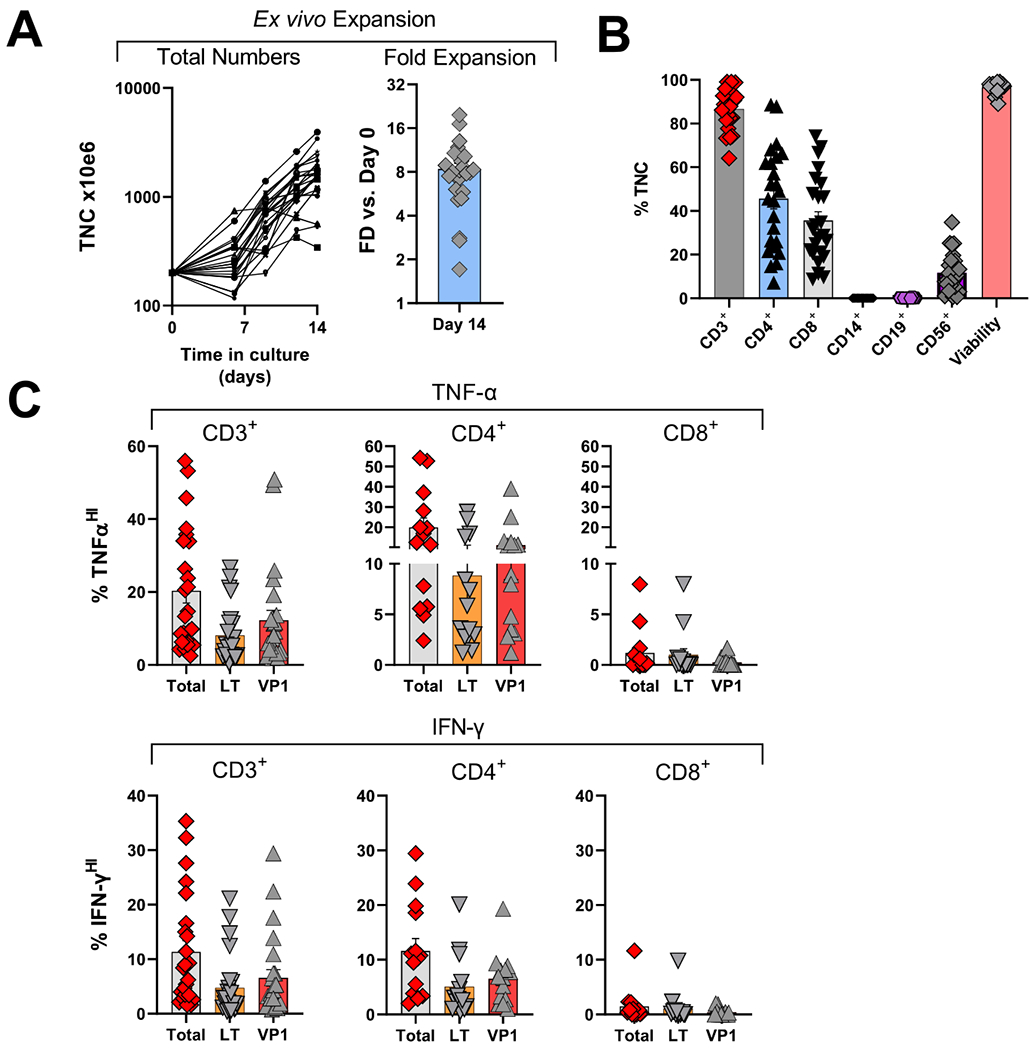
Characterization of clinical allogeneic PyVST products derived from matched healthy donors.
Peripheral blood mononuclear cells from healthy related donors were isolated from the apheresis product, stimulated with BK virus LT and VP1 pepmixes and expanded in G-rex tissue culture containers for 14 days. Upon harvest, as part of the release criteria, the resulting PyVST were enumerated, analyzed for identity, cellular content, and viability. Additionally, antiviral potency was assessed, measured by cytokine release. A. Expansion of PyVST cells as measured by absolute number of viable nucleated cells (left panel) and fold expansion relative to day 0 from the initiation of the culture (right panel). B. Composition and viability of the final PyVST products was evaluated by flow cytometry and trypan blue exclusion (respectively) on harvest (day 14 from the culture initiation). C. Antiviral activity of the final PyVST products in the viable CD3+, CD4+, and CD8+ T cell compartments was measured by intracellular staining for TNF-α and IFN-γ upon 6hr restimulation with BK virus LT and/or VP1 pepmixes in presence of brefeldin A and monesin using flow cytometry (infused products only shown). Unrelated pepmix was used as negative control.
