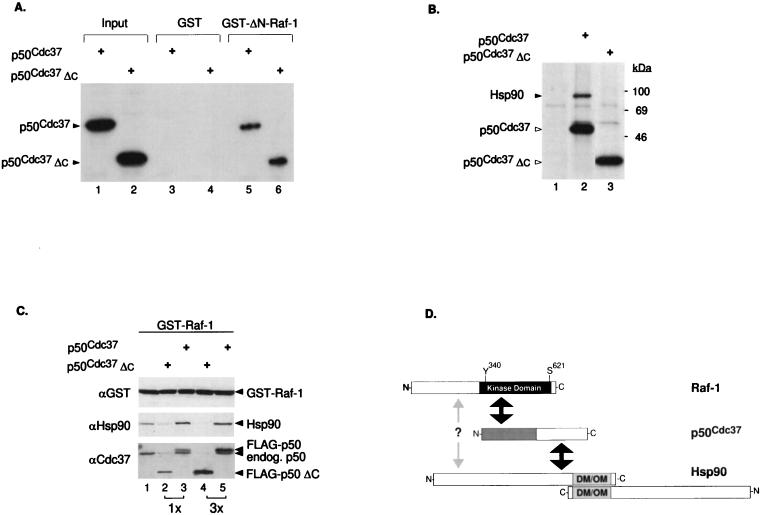
FIG. 2.
The N-terminal half of p50cdc37 mediates association with the catalytic domain of Raf-1 but is impaired for Hsp90 interaction and accumulation to Raf-1. (A) Plasmids pSG5-p50cdc37 and pSG5-p50cdc37ΔC were transcribed and translated in vitro, using T7 RNA polymerase and a reticulocyte lysate system (Promega); 5 μl of each reaction mixture was either analyzed directly (input lanes) or assayed in vitro for binding to either GST or bacterially purified GST–ΔN-Raf-1(Δ26-309) and visualized by SDS-PAGE and fluorography. Comparable results were obtained with full-length GST–Raf-1 (not shown). (B) Cos-1 cells transfected with pSG5-FLAG vector, pSG5-FLAG-p50cdc37, and pSG5-FLAG-p50cdc37ΔC were [35S]methionine labeled, and anti-FLAG IPs in NP-40 LB of each transfected sample were analyzed by SDS-PAGE and fluorography (lanes 1 to 3, respectively). Proteins at the sizes predicted for overexpressed FLAG-p50cdc37 proteins or associated endogenous Hsp90 are also indicated. (C) Two micrograms of pEBG-GST-Raf-1 was cotransfected with 5 μg of pSG5-FLAG vector (lane 1), pSG5-FLAG-p50cdc37 (lanes 2 and 3), or pSG5-FLAG-p50cdc37ΔC (lanes 4 and 5) at 5 or 15 μg as indicated. After 48 h in DMEM-FBS, all five cultures were harvested and lysed in NP-40 LB, and GST–Raf-1 was GSH-Sepharose purified and tested for associated p50cdc37 or Hsp90 proteins with rabbit anti-p50cdc37 or rat anti-Hsp90 antibody. A control anti-GST immunoblot was also included to detect overexpressed GST–Raf-1 (top panel). (D) Diagram indicating regions of interaction between p50cdc37, Raf-1, and Hsp90. The N-terminal half of p50cdc37 (gray area) which corresponds to p50cdc37ΔC is sufficient for interacting with the C-terminal kinase domain of Raf-1, while its C-terminal half mediates Hsp90 interaction (indicated by black arrows). A distinct weak interaction of Raf-1 directly with Hsp90 through as yet unidentified domains is also proposed and is indicated by the gray arrow. Relative positions of the Y340 and S621 phosphorylation sites present on Raf-1 are also indicated. Since Hsp90 can both homodimerize and form oligomers through its C terminus (DM/OM) (41, 48, 49), higher-order complexes of p50cdc37–Raf-1–Hsp90 can also be envisioned.