Figure 3.
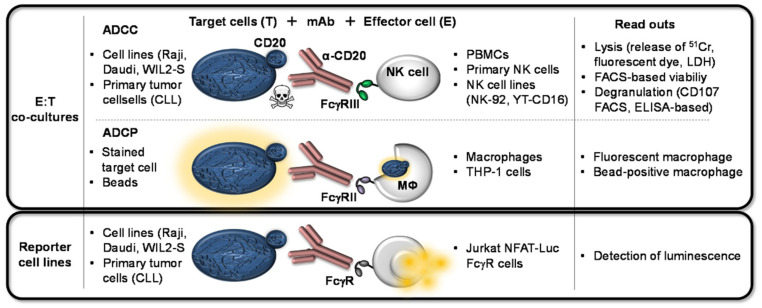
Cell-based assays used to assess the FcγR-mediated effector functions can be classified into co-culture systems involving effector plus target cells (E:T co-cultures) and assays including reporter cell lines. For the analyses of α-CD20-induced ADCC, several cell lines or primary tumor cells (such as Chronic Lymphatic Leukemia (CLL) cells) expressing CD20 can serve as target cells. Effector cell cultures for such assays can comprise crude PBMC cell cultures, primary NK cells, or NK cell lines. NK cell-mediated killing upon α-CD20:FcγRIIIa crosslinking can be detected via released labeling components in the cell culture supernatant (pre-labeled 51Cr, fluorescent dyes, or release of internal enzymes such as lactate dehydrogenase (LDH)). Further ADCC read outs are the detection of viability via FACS-based analyses or determining degranulation (CD107 by FACS analyses or ELISA). For the analyses of α-CD20-induced ADCP also several cell lines or primary tumor cells are used as target cells. FcγRIIa-expressing macrophages or macrophage-like cell lines (e.g., THP-1) are used as effector cells, which increase phagocytosis upon FcγRIIa-crosslinking with the mAb. When the target cells are pre-labeled with a dye or beads, their depletion can be traced by detecting fluorescent or bead-positive macrophages. Within reporter cell-based assays, the CD20-expressing target cells are co-cultured with Jurkat NFAT-Luc cells and the α-CD20 mAbs. When using this reporter cell lines, the FcγR-mediated effect upon crosslinking is replaced by the secretion of luciferase and can hence be determined by the intensity of luminescence.