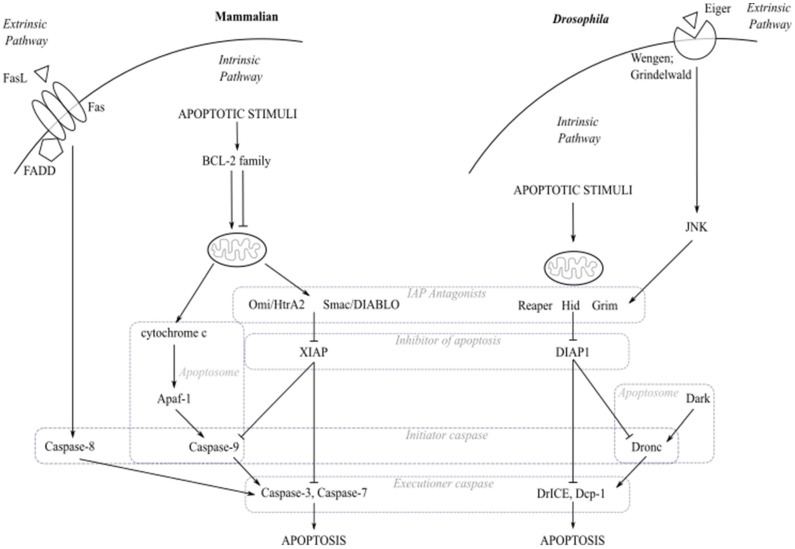
Figure 1.
The core apoptosis pathway in mammals and Drosophila. During activation of the intrinsic apoptosis pathway in mammalian cells, apoptotic stimuli cause expression of pro-apoptotic BCL-2 family members resulting in the perturbation of the mitochondrial outer membrane and release of small molecules including cytochrome c, Omi/HtrA2 and Smac/DIABLO. Cytochrome c binds to Apaf-1 forming the apoptosome which recruits the pro-caspase-9 leading to its activation. Meanwhile, Omi/HtrA2 and Smac/DIABLO act as IAP antagonists to free caspases from the inhibition of apoptotic proteins such as XIAP. Active caspase-9 cleaves and activates caspase-3 and -7, allowing execution of apoptosis. The mammalian extrinsic apoptosis pathway is activated upon ligand binding to death receptor, e.g., Fas. Adaptor protein FADD recruitment to Fas forms the death-inducing signalling complex (DISC) which binds and activates caspase-8. Active caspase-8 cleaves and activates caspase-3 and caspase-7, allowing execution of apoptosis. In the intrinsic apoptosis pathway in Drosophila, apoptotic stimuli activate expression of pro-apoptotic proteins such as Reaper, Hid and Grim which need to localise to the mitochondria to become fully activated. These pro-apoptotic proteins release caspases from DIAP1 held inhibition. The initiator caspase Dronc then associates with the scaffold protein Dark to form an apoptosome-like complex. Active Dronc cleaves executioner caspases, DrICE and Dcp-1, which bring about apoptosis. The Drosophila extrinsic apoptosis pathway is activated by binding of Eiger to the death receptors Wengen or Grindelwald, resulting in activation of the JNK signalling pathway. Active JNK signalling induces expression of pro-apoptotic proteins, Hid and Reaper. The evolutionarily conserved core components in the apoptosis pathway are highlighted with the dashed line boxes.