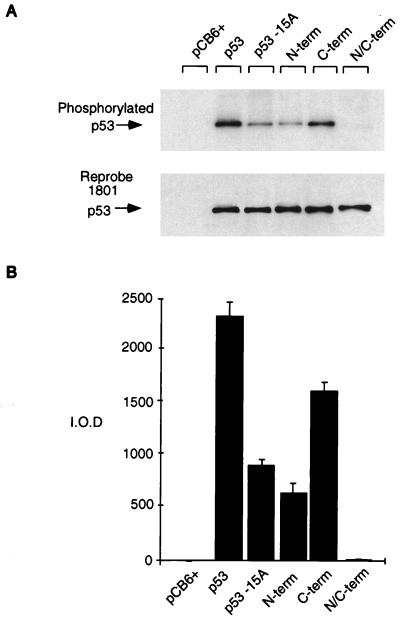
FIG. 2.
Relative phosphorylation of p53 mutant proteins in vivo. Phosphorylation site mutant p53 proteins (5 μg) were transiently transfected into Saos-2 cells. At approximately 4 h prior to harvesting (at 24 h), the cells were washed and incubated in 5 ml of phosphate-free medium (supplemented with 10% dialyzed FCS) containing 1 mCi of 32P-labeled sodium orthophosphate (per 10-cm2 dish). Cells were lysed with radioimmunoprecipitation assay lysis buffer, and p53 protein was immunoprecipitated with PAb1801-protein A Sepharose beads. (A) Phosphorylated protein was assessed by SDS–10% PAGE (top), and blots were reprobed with PAb1801 to assess immunoprecipitated p53 levels (bottom). (B) Phosphorylated protein was quantified by PhosphorImager analysis and represented graphically by using absolute integrated optical density units (I.O.D.). The graph represents the results of four independent experiments.