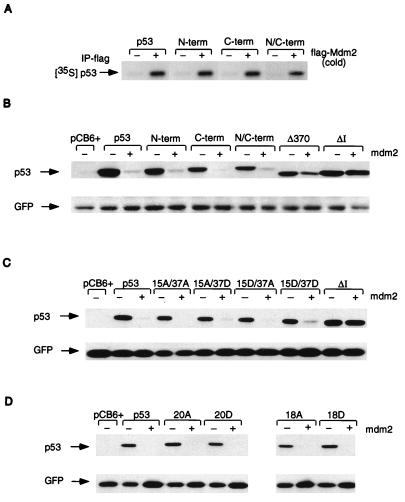
FIG. 5.
(A) In vitro association assay using in vitro-translated, unlabeled, human flag-Mdm2 (cold) and 35S-labeled, in vitro-translated wild-type p53 and p53N-term, p53C-term, and p53N/C-term mutant proteins. Complexes were immunoprecipitated (IP) with anti-flag antibody in the presence of protein G Sepharose beads, and radioactively labeled p53 proteins were assessed by SDS–10% PAGE. (B) Western blot analysis (using PAb1801) of Saos-2 cells after transient cotransfection of the phosphorylation mutant p53 (3 μg), mouse Mdm2 (9 μg), and pEGFP-N1 (1 μg). Cells were harvested at 24 h. The ΔI mutant protein (deletion of conserved box I, the region containing the Mdm2 binding site), which is resistant to Mdm2-mediated degradation, and the Δ370 mutant protein (C-terminal truncation mutant protein), which is partially resistant to Mdm2-mediated degradation, were used as controls. Relative transfection efficiency was monitored by expression of cotransfected GFP. (C) Western blot analysis (using PAb1801) of Saos-2 cells after transient cotransfection of the p53-15A/37A, -15A/37D, -15D/37A, and -15D/37D combination mutant proteins (3 μg), mouse Mdm2 (9 μg), and pEGFP-N1 (1 μg). Cells were harvested at 24 h. Relative transfection efficiency was monitored by expression of cotransfected GFP. (D) Western blot analysis (using PAb1801) of Saos-2 cells after transient cotransfection of the p53-20A, -20DD, -18, and -18D combination mutant proteins (3 μg), mouse Mdm2 (9 μg), and pEGFP-N1 (1 μg). Cells were harvested at 24 h. Relative transfection efficiency was monitored by expression of cotransfected GFP.