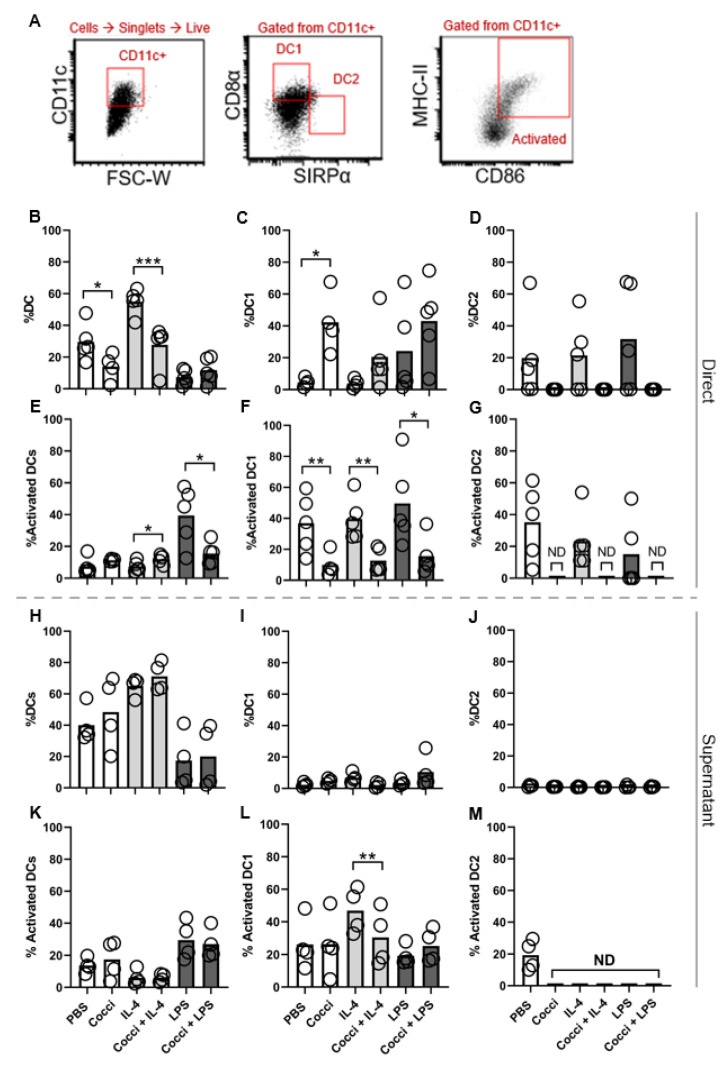
Figure 3.
DCs polarize to DC1 in response to Coccidioides but do not upregulate CD86 and MHC-II expression. (A) Representative flow plots show gating strategy used for analysis; sample shown is stimulated with Coccidioides. See Supplemental Figure S5 for complete gating strategy. Using a C57BL/6 bone marrow-derived DC culture system to generate DCs, we assessed DC polarization. Unstim refers to DC culture conditions without cytokine stimulation. LPS (1 µg/mL) was added as a DC1 control and IL-4 (0.5 ng/mL) as a DC2 control. Coccidioides was added in a 1:1 ratio with cells. (B–G) DCs were directly stimulated with Coccidioides, LPS and/or IL-4. (H–M) DCs were stimulated using supernatants generated in (B–G). (B,H) DC frequency of all CD11c+ cells including DC1 and DC2. (C,D,I,J) DC1 and DC2 populations were gated from total CD11c+ population. (C,F,I,L) DC1 are defined as CD8α+SIRPα-CD11c+ and (D,G) DC2 as CD8a-SIRPa+CD11c+. (E–G,K–M) Activated cells are defined as CD86+MHC-II+; activated cells are gated from their respective subtype population. ND = not determined due to lack of cells from previous gate. n = 4–5, data are representative of 5 experiments for direct assay, and 3 experiments for supernatant assay. The bar within each data group indicates the mean, each individual circle represents one biological replicate averaged from all technical replicates within each experiment. Statistics show comparisons between Coccidioides and non-Coccidioides conditions. Data was analyzed using unpaired Student’s t-test and outliers excluded using Grubbs Outlier exclusion analysis * p < 0.05, ** p < 0.005. *** p < 0.0005.