Fig. 5. Mechanistic studies.
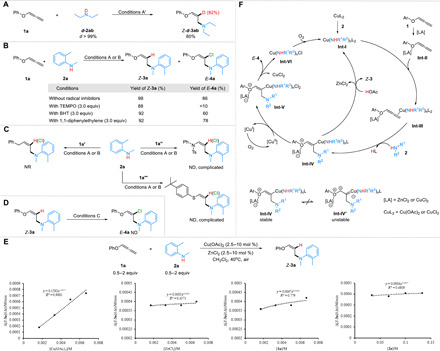
(A) Deuterium-labeling study. (B) Radical trapping experiment. (C) Control experiment. (D) Intermediate experiment. (E) Determination of the order. (F) Proposed mechanism. Condition A′: 1a (0.3 mmol), d-2ab (0.2 mmol), Cu(OAc)2 (5 mol %), ZnCl2 (5 mol %), and dry CH2Cl2 (1.0 ml), 50°C, 6 hours. Condition A: 1 (0.3 mmol), 2a (0.2 mmol), Cu(OAc)2 (5 mol %), ZnCl2 (5 mol %), and CH2Cl2 (1.0 ml), 50°C, 6 hours. Condition B: 1 (0.3 mmol), 2a (0.2 mmol), CuCl2 (60 mol %), dioxane (1.0 ml), and O2 (1 atm), 40°C, 6 hours. Condition C: Z-3a (0.3 mmol), CuCl2 (60 mol %), dioxane (1.0 ml), and O2 (1 atm), 40°C, 6 hours. 1a′: Buta-2,3-dien-1-ylbenzene. 1a″: 4-Methyl-N-phenyl-N-(propa-1,2-dien-1-yl)benzenesulfonamide. 1a‴: 1-tert-Butyl-4-(propa-1,2-dienylthio)benzene.
