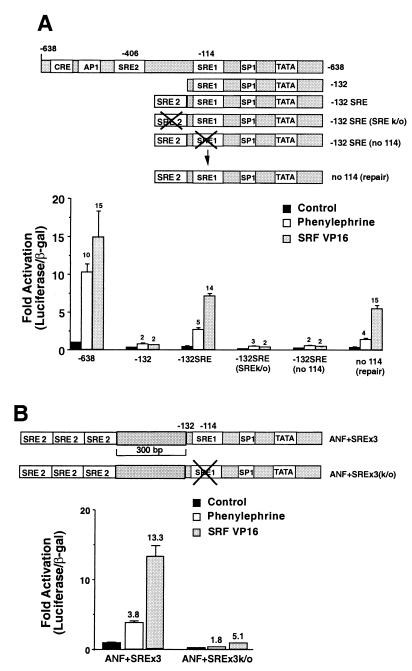
FIG. 4.
Cooperation between SRE2 and SRE1. (A) The wild-type −638 promoter or the −132 truncation (−132), the −132 promoter with a functional SRE2 element fused to the end (−132 SRE), −132 mutants containing mutated SRE2 (SREk/o) or SRE1 (no114), or repairs of the mutated sequence (no 114 repair) were induced by phenylephrine or SRF-VP16. All transfections were normalized to the untreated controls for each promoter. The fold activation for each promoter (from its unstimulated control) is given by the numbers above the bars. Note that the −132 promoter is not significantly activated by either phenylephrine or SRF-VP16, while the promoter with a single copy of the SRE2 element fused to the end of −132 regains wild-type activation by SRF-VP16 and partially rescues activation by phenylephrine. Mutation of either SRE element reduces activation by both stimuli, while remutation to repair the SRE1 mutant rescues activation by both stimuli. (B) Three SRE2 elements were fused to the end of a 300-bp DNA fragment from the Kan gene on the end of the −132 promoter. The SRE1 element was mutated in this construct, and stimulation was assessed after treatment with phenylephrine or SRF-VP16. Note that mutation of the SRE1 element reduces basal promoter activity and induction by both phenylephrine and SRF-VP16.