Key Points
Question
Are early estimates of household transmission of SARS-CoV-2 indicative of current household transmission?
Findings
In this updated systematic review and meta-analysis of 87 studies representing 1 249 163 household contacts from 30 countries, the estimated household secondary attack rate was 19%. An increase in household transmission was observed over time, perhaps owing to improved diagnostic procedures and tools, longer follow-up, more contagious variants, and different study locations.
Meaning
These findings suggest that the household remains an important site of SARS-CoV-2 transmission, and recent studies have generated higher household secondary attack rate estimates compared with the earliest reports; more transmissible variants and vaccines may be associated with additional changes in the future.
Abstract
Importance
A previous systematic review and meta-analysis of household transmission of SARS-CoV-2 that summarized 54 published studies through October 19, 2020, found an overall secondary attack rate (SAR) of 16.6% (95% CI, 14.0%-19.3%). However, the understanding of household secondary attack rates for SARS-CoV-2 is still evolving, and updated analysis is needed.
Objective
To use newly published data to further the understanding of SARS-CoV-2 transmission in the household.
Data Sources
PubMed and reference lists of eligible articles were used to search for records published between October 20, 2020, and June 17, 2021. No restrictions on language, study design, time, or place of publication were applied. Studies published as preprints were included.
Study Selection
Articles with original data that reported at least 2 of the following factors were included: number of household contacts with infection, total number of household contacts, and secondary attack rates among household contacts. Studies that reported household infection prevalence (which includes index cases), that tested contacts using antibody tests only, and that included populations overlapping with another included study were excluded. Search terms were SARS-CoV-2 or COVID-19 with secondary attack rate, household, close contacts, contact transmission, contact attack rate, or family transmission.
Data Extraction and Synthesis
Meta-analyses were performed using generalized linear mixed models to obtain SAR estimates and 95% CIs. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline was followed.
Main Outcomes and Measures
Overall household SAR for SARS-CoV-2, SAR by covariates (contact age, sex, ethnicity, comorbidities, and relationship; index case age, sex, symptom status, presence of fever, and presence of cough; number of contacts; study location; and variant), and SAR by index case identification period.
Results
A total of 2722 records (2710 records from database searches and 12 records from the reference lists of eligible articles) published between October 20, 2020, and June 17, 2021, were identified. Of those, 93 full-text articles reporting household transmission of SARS-CoV-2 were assessed for eligibility, and 37 studies were included. These 37 new studies were combined with 50 of the 54 studies (published through October 19, 2020) from our previous review (4 studies from Wuhan, China, were excluded because their study populations overlapped with another recent study), resulting in a total of 87 studies representing 1 249 163 household contacts from 30 countries. The estimated household SAR for all 87 studies was 18.9% (95% CI, 16.2%-22.0%). Compared with studies from January to February 2020, the SAR for studies from July 2020 to March 2021 was higher (13.4% [95% CI, 10.7%-16.7%] vs 31.1% [95% CI, 22.6%-41.1%], respectively). Results from subgroup analyses were similar to those reported in a previous systematic review and meta-analysis; however, the SAR was higher to contacts with comorbidities (3 studies; 50.0% [95% CI, 41.4%-58.6%]) compared with previous findings, and the estimated household SAR for the B.1.1.7 (α) variant was 24.5% (3 studies; 95% CI, 10.9%-46.2%).
Conclusions and Relevance
The findings of this study suggest that the household remains an important site of SARS-CoV-2 transmission, and recent studies have higher household SAR estimates compared with the earliest reports. More transmissible variants and vaccines may be associated with further changes.
This systematic review and meta-analysis combines data from a previous meta-analysis of SARS-CoV-2 household transmission through October 19, 2020, with new data from studies published between October 20, 2020, and June 17, 2021, to provide updated estimates of household secondary attack rates.
Introduction
Understanding of the household secondary attack rate for SARS-CoV-2 is still evolving. We previously published a systematic review and meta-analysis of household transmission of SARS-CoV-2 that summarized 54 published studies representing 77 758 household contacts through October 19, 2020, finding an overall secondary attack rate (SAR) of 16.6% (95% CI, 14.0%-19.3%).1 Household SARs were higher to adult contacts than to child contacts, to spouses than to other contacts, from symptomatic index cases than from asymptomatic index cases, and in households with 1 contact than in households with 3 or more contacts. The SARs were higher to household contacts than to other close contacts. Household SARs were also higher for SARS-CoV-2 than for SARS-CoV and Middle East respiratory syndrome coronavirus. This living systematic review and meta-analysis updated those findings through June 17, 2021, and used newly published data to further our understanding of the household’s role in SARS-CoV-2 transmission.2
Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline using the same definitions, search strategy, eligibility criteria, and data extraction methods used in our original study.1 We searched PubMed and reference lists of eligible articles for studies published between October 20, 2020, and June 17, 2021, with no restrictions on language, study design, time, or place of publication. Studies published as preprints were included. Search terms were SARS-CoV-2 or COVID-19 with secondary attack rate, household, close contacts, contact transmission, contact attack rate, or family transmission.
Articles with original data that reported at least 2 of the following factors were included: number of household contacts with infection, total number of household contacts, and secondary attack rates among household contacts. Studies that reported household infection prevalence (including index cases), that tested contacts using antibody tests only, and that included populations that overlapped with another included study were excluded.
In addition to the covariates examined previously, we also examined SAR by contact ethnicity (restricted to studies in the US), contact comorbidity, index case fever, index case cough, and variant (if reported in ≥3 studies). Primary outcomes were overall household SAR for SARS-CoV-2, SAR by covariates (contact age, sex, ethnicity, comorbidities, and relationship; index case age, sex, symptom status, presence of fever, and presence of cough; number of contacts; study location; and variant), and SAR by index case identification period. We categorized contact and index case age as adults (aged ≥18 years) and children (aged <18 years). For studies that reported SARs by age using 10-year increments (eg, 10-19 years), we included those aged 18 and 19 years in the child category. For the symptom status of the index case covariate, we included studies that disaggregated SARs for at least 2 of the following: symptomatic, presymptomatic, and asymptomatic individuals. We also conducted a sensitivity analysis restricted to studies with a more uniform design, which excluded studies with only asymptomatic or pediatric index cases, studies that tested only symptomatic or asymptomatic contacts, studies with long follow-up periods (≥21 days), and studies published as preprints.
In addition, to examine temporal patterns, we assessed household SARs by index case identification period (January-February 2020, March-April 2020, May-June 2020, and July 2020-March 2021). If the study period spanned multiple months, we used the midpoint. For example, when the index case identification period for all households was December 2019 to April 2020, the midpoint was February 2020, and the study was categorized as January to February 2020.
Statistical Analysis
Statistical analyses were similar to those previously described.1 However, this analysis used generalized linear mixed models to obtain SAR estimates and 95% CIs; these models appear to be more robust for meta-analyses of single proportions compared with Freeman-Tukey double arcsine transformation.3 Heterogeneity was measured using the I2 statistic, with thresholds of 25%, 50%, and 75% indicating low, moderate, and high heterogeneity, respectively. All analyses were performed using the metafor package in R software, version 4.0.2 (R Foundation for Statistical Computing). Statistical significance was set at 2-tailed P = .05.
Results
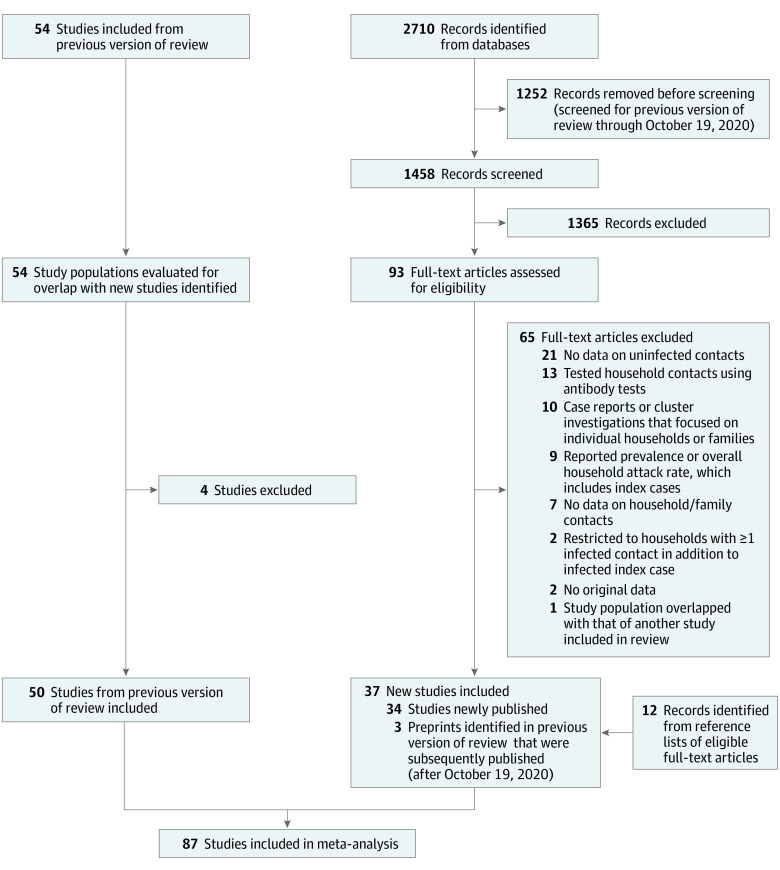
We identified 2722 records (2710 records from database searches and 12 records from the reference lists of eligible articles) published between October 20, 2020, and June 17, 2021; of those, 93 full-text articles reporting household secondary transmission of SARS-CoV-2 were assessed for eligibility, and 37 studies4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40 were eligible for inclusion (3 of these studies were preprints that were identified in our previous review and subsequently published) (Figure 1; eTable 1 in the Supplement). These 37 new studies were combined with 50 of the 54 studies (published through October 19, 2020) included in our previous review (4 studies41,42,43,44 from Wuhan, China, were excluded because their study populations overlapped with another recent study),14 resulting in 87 total studies4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94 representing 1 249 163 household contacts from 30 countries. The estimated overall household SAR for all 87 studies was 18.9% (95% CI, 16.2%-22.0%), with significant heterogeneity (I2 = 99.4%; P < .001) (Figure 2). Excluding studies with only asymptomatic85 or pediatric36,66 index cases, studies that tested only7,9,15,17,19,24,26,29,30,31,35,37,45,47,61,65,68,69,71,77,79,81,82,86,87,90,92,94 or asymptomatic78 contacts, studies with long follow-up periods (≥21 days),5,8,9,23,46,92 and studies published as preprints,8,23,24,29,45,79,88,89,90,92 the overall SAR among the 47 remaining studies4,6,10,11,12,13,14,16,18,20,21,22,25,27,28,32,33,34,38,39,48,49,50,51,52,53,54,55,57,58,59,60,62,63,64,67,70,72,73,74,75,76,80,83,84,91,93 was 19.9% (95% CI, 16.2%-24.2%).
Figure 1. PRISMA Flow Diagram.
Figure 2. Household Secondary Attack Rates by Study Location.
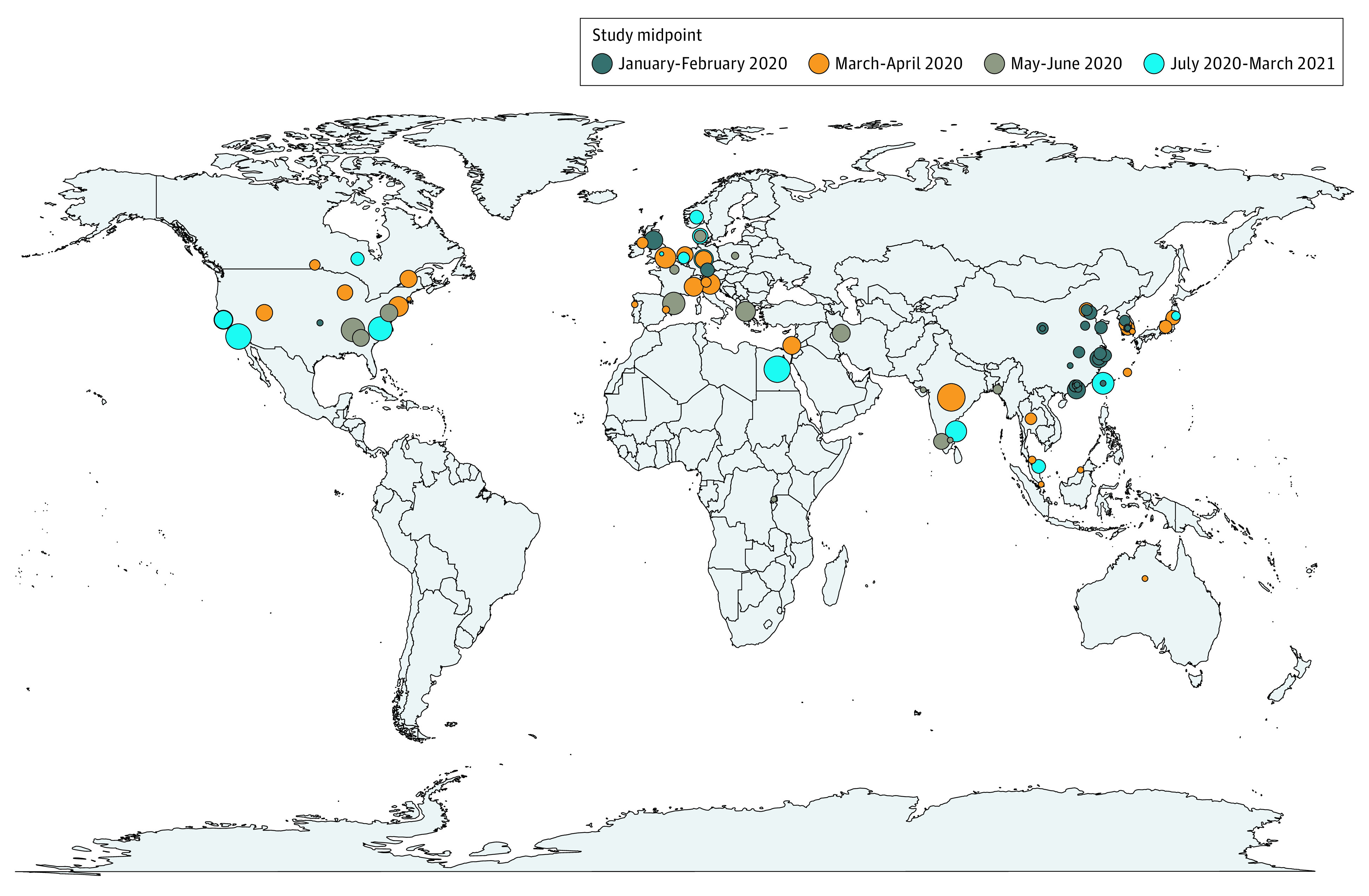
For studies that included data from multiple regions within a country, a point in the center of the country was selected. Circle sizes represent extent of secondary attack rates, with small circles indicating 0.2, medium circles indicating 0.4, and large circles indicating 0.6.
When analyzing household SAR by study period, we observed an increasing pattern over time. Compared with the SAR for 28 studies12,14,17,27,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,94 from January to February 2020 (13.4%; 95% CI, 10.7%-16.7%), the SAR was significantly higher for 30 studies6,7,15,16,19,22,25,26,28,30,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,93 from March to April 2020 (19.4%; 95% CI, 15.2%-24.5%; P = .03) and 15 studies5,8,10,18,20,21,23,24,29,31,32,35,37,38,40 from July 2020 to March 2021 (31.1%; 95% CI, 22.6%-41.1%; P < .001) but not significantly different from the SAR for 14 studies4,9,11,13,33,34,36,39,87,88,89,90,91,92 from May to June 2020 (19.9%; 95% CI, 13.0%-29.3%; P = .07) (Figure 314). To elucidate factors associated with differences in SAR, we explored attributes of studies from the periods with the lowest and highest household SARs. Among 28 studies12,14,17,27,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,94 from January to February 2020 and 15 studies5,8,10,18,20,21,23,24,29,31,32,35,37,38,40 from July 2020 to March 2021, 6 studies12,46,54,57,59,62 (21.4%) and 4 studies8,10,20,23 (25.0%), respectively, reported testing contacts at least twice, 1 study46 (3.6%) and 3 studies5,8,23 (18.8%) reported following contacts for longer than 14 days, 1 study45 (3.6%) and 6 studies8,23,24,29,37,40 (33.3%) were published as preprints, 21 studies12,14,27,46,48,49,50,51,52,53,54,55,57,58,59,60,62,63,64,66,67 (75.0%) and 10 studies5,8,10,18,20,21,23,32,38,40 (66.6%) tested all contacts regardless of symptoms, and 0 studies and 3 studies18,35,40 (18.8%) reported SARs for variants of concern (VOCs).
Figure 3. Household Secondary Attack Rates by Midpoint of Index Case Identification Period.
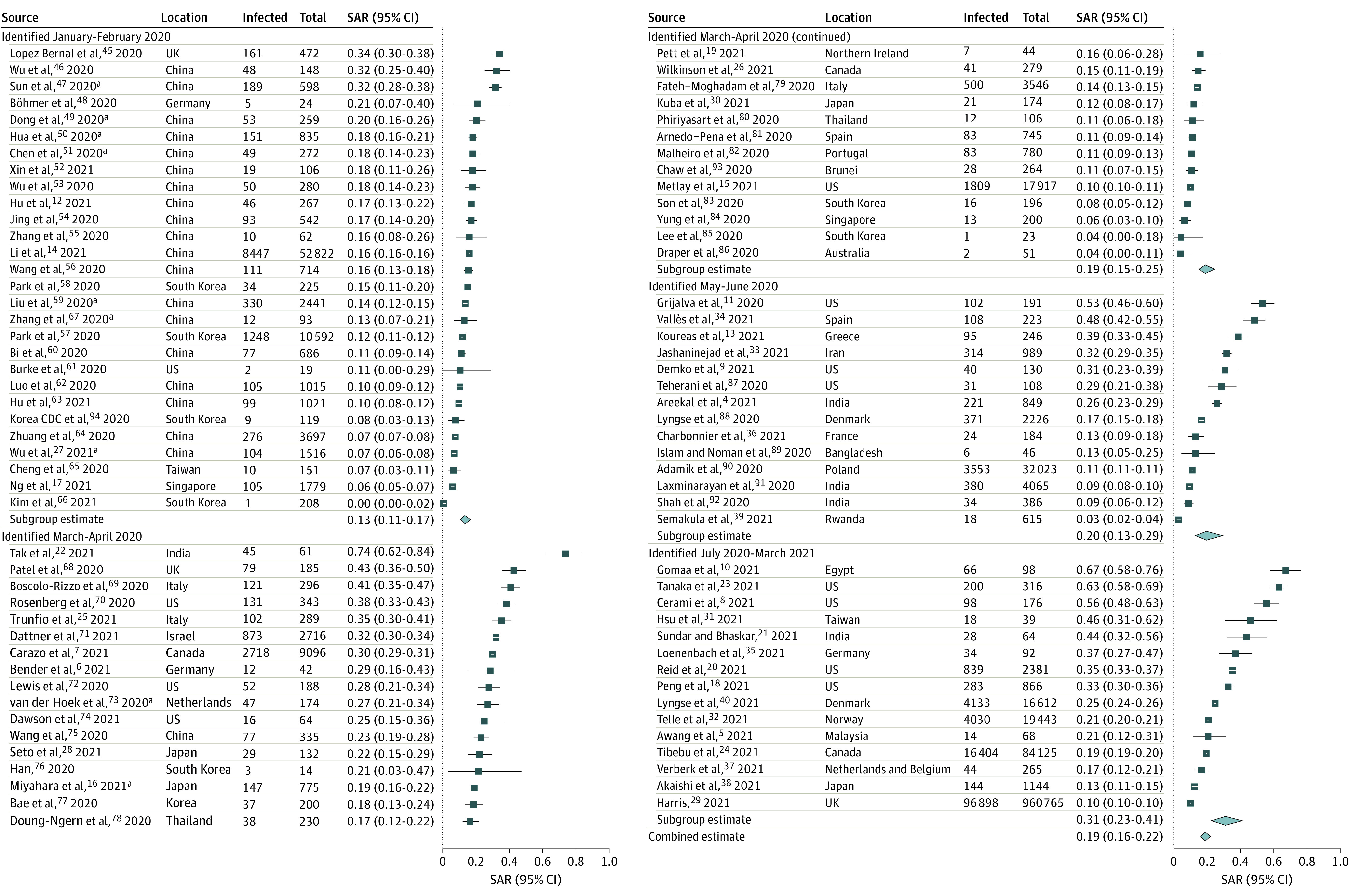
For studies that spanned multiple months, the midpoint was used. For example, when the index case identification period for all households was December 2019 to April 2020, the midpoint was February 2020, and the study was categorized as January to February 2020. The meta-analysis excluded 4 studies from Wuhan, China,41,42,43,44 that had overlapping populations with Li et al.14 Point sizes are an inverse function of the precision of the estimates, and bars correspond to 95% CIs. Diamonds represent summary SAR estimates with corresponding 95% CIs.
aStudy included family contacts, which may have comprised individuals outside the household.
The SARs were significantly higher for adult contacts (29.9%; 95% CI, 24.0%-36.6%) than for child contacts (17.5%; 95% CI, 12.6%-23.7%; P < .001),7,8,11,13,14,15,26,30,32,35,40,45,46,50,54,60,70,71,72,73,75,87,88,91 for spousal contacts (39.8%; 95% CI, 30.0%-50.5%) than for other household contacts (18.3%; 95% CI, 12.1%-26.7%; P = .001),8,11,17,30,33,46,47,52,72,93,95 for contacts with comorbidities (50.0%; 95% CI, 41.4%-58.6%) than for contacts without comorbidities (22.0%; 95% CI, 13.4%-33.9%; P = .04),30,45,46 in symptomatic index cases (20.2%; 95% CI, 13.9%-28.3%)6,13,14,16,24,27,58,93 than in asymptomatic (3.0%; 95% CI, 1.7%-5.4%)6,14,24,27,58,93 or presymptomatic (8.1%; 95% CI, 7.3%-9.1%; P < .001)24,58,93 index cases, and in households with 1 contact (35.5%; 95% CI, 26.2%-46.2%) than in households with 3 or more contacts (21.2%; 95% CI, 14.8%-29.4%; P = .02)11,16,30,32,40,41,45,46,70,81,88 (Table). The SARs were not associated with the contact’s sex8,11,13,14,15,17,26,28,30,33,40,45,46,47,52,54,72,81,84,88,91 or ethnicity11,18,72 or with the index case’s age,11,13,14,16,24,32,35,57,91 sex,11,13,14,16,24,32,46,52,72,81,84,91 presence of fever,11,46,52 or presence of cough.11,46,52 When the analysis was restricted to laboratory-confirmed results,30,45,46 the estimated SAR to contacts with comorbidities was 43.9% (95% CI, 32.1%-56.5%). The estimated mean SAR for the B.1.1.7 (α) variant was 24.5% (95% CI, 10.9%-46.2%),35,40,96 with significant heterogeneity (I2 = 99.5%; P < .001) (eFigure in the Supplement). Restricting the analysis to studies with a more uniform design,11,16,32,70 SARs were not significantly different for the number of contacts in the household (P = .51) (eTable 2 in the Supplement). No studies with data regarding the comorbidity covariate met the criteria for inclusion in this subanalysis.
Table. Characteristics of Studies Included in Analysis of Household Secondary Attack Rates for SARS-CoV-2.
| Characteristic | Studies, No. | SAR, % (95% CI) |
|---|---|---|
| Measures used for overall SAR assessment | ||
| Laboratory-confirmed results plus probable untested symptomatic cases | 874,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94a | 18.9 (16.2-22.0) |
| Laboratory-confirmed results only | 814,5,6,8,9,10,11,12,13,14,15,16,17,18,20,21,22,23,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,69,70,71,72,73,74,75,76,77,78,79,80,82,83,84,85,86,88,89,90,91,92,93,94a | 18.1 (15.4-21.3) |
| Contact age | ||
| Adults (≥18 y) | 247,8,11,13,14,15,26,30,32,35,40,45,46,50,54,60,70,71,72,73,75,87,88,91b | 29.9 (24.0-36.6) |
| Children (<18 y) | 247,8,11,13,14,15,26,30,32,35,40,45,46,50,54,60,70,71,72,73,75,87,88,91b | 17.5 (12.6-23.7) |
| Contact sex | ||
| Female | 218,11,13,14,15,17,26,28,30,33,40,45,46,47,52,54,72,81,84,88,91b | 22.4 (17.4-28.5) |
| Male | 218,11,13,14,15,17,26,28,30,33,40,45,46,47,52,54,72,81,84,88,91b | 20.2 (15.2-26.4) |
| Contact ethnicityc | ||
| Hispanic or Latino | 311,18,72 | 36.0 (16.7-61.2) |
| Non-Hispanic or non-Latino | 311,18,72 | 36.4 (25.7-48.8) |
| Contact comorbidities | ||
| Any | 330,45,46 | 50.0 (41.4-58.6) |
| None indicated | 330,45,46 | 22.0 (13.4-33.9) |
| Relationship to index case | ||
| Spouse | 118,11,17,30,33,46,47,52,72,93,95 | 39.8 (30.0-50.5) |
| Other | 118,11,17,30,33,46,47,52,72,93,95 | 18.3 (12.1-26.7) |
| Index case age | ||
| Adult (≥18 y) | 911,13,14,16,24,32,35,57,91 | 22.7 (15.2-32.6) |
| Child (<18 y) | 911,13,14,16,24,32,35,57,91 | 18.5 (11.8-27.7) |
| Index case sex | ||
| Female | 1211,13,14,16,24,32,46,52,72,81,84,91b | 22.3 (15.8-30.5) |
| Male | 1211,13,14,16,24,32,46,52,72,81,84,91b | 21.3 (15.1-29.2) |
| Index case symptom statusd | ||
| Symptomatic | 86,13,14,16,24,27,58,93 | 20.2 (13.9-28.3) |
| Asymptomatic | 66,14,24,27,58,93 | 3.0 (1.7-5.4) |
| Presymptomatic | 324,58,93 | 8.1 (7.3-9.1) |
| Asymptomatic and/or presymptomatic | 86,13,14,16,24,27,58,93 | 3.9 (2.1-6.8) |
| Index case fever | ||
| Yes | 311,46,52 | 20.6 (12.2-32.7) |
| No | 311,46,52 | 14.7 (10.6-19.9) |
| Index case cough | ||
| Yes | 311,46,52 | 22.7 (11.3-40.3) |
| No | 311,46,52 | 17.3 (13.9-21.4) |
| Contacts in household, No. | ||
| 1 | 1111,16,30,32,40,41,45,46,70,81,88 | 35.5 (26.2-46.2) |
| 2 | 1111,16,30,32,40,41,45,46,70,81,88 | 31.8 (20.4-45.9) |
| ≥3 | 1111,16,30,32,40,41,45,46,70,81,88 | 21.2 (14.8-29.4) |
| Location | ||
| China or Singapore | 2212,14,17,27,46,47,49,50,51,52,53,54,55,56,59,60,62,63,64,67,75,84a | 14.4 (11.8-17.4) |
| Other | 654,5,6,7,8,9,10,11,13,15,16,18,19,20,21,22,23,24,25,26,28,29,30,31,32,33,34,35,36,37,38,39,40,45,48,57,58,61,65,66,68,69,70,71,72,73,74,76,77,78,79,80,81,82,83,85,86,87,88,89,90,91,92,93,94 | 20.7 (17.0-24.9) |
| Testing protocole | ||
| Symptomatic and asymptomatic individuals | 574,5,6,8,10,11,12,13,14,16,18,20,21,22,23,25,27,28,32,33,34,36,38,39,40,46,48,49,50,51,52,53,54,55,57,58,59,60,62,63,64,66,67,70,72,73,74,75,76,80,83,84,85,88,89,91,93a | 19.8 (16.1-24.1) |
| Symptomatic individuals only | 287,9,15,17,19,24,26,29,30,31,35,37,45,47,61,65,68,69,71,77,79,81,82,86,87,90,92,94a | 17.5 (13.6-22.1) |
| Index case identification period excluding overlapping dates | ||
| December 2019-April 2020 | 526,12,14,17,19,22,25,26,27,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,93,94a | 15.8 (13.0-19.1) |
| July 2020-March 2021 | 144,5,18,20,21,23,24,29,33,34,35,36,38,88 | 27.7 (20.6-36.2) |
| Study published as preprint | ||
| Yes | 128,23,24,29,37,40,45,79,88,89,90,92 | 21.0 (13.8-30.6) |
| No | 754,5,6,7,9,10,11,12,13,14,15,16,17,18,19,20,21,22,25,26,27,28,30,31,32,33,34,35,36,37,38,39,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,74,75,76,77,78,80,81,82,83,84,85,86,87,91,93,94 | 18.6 (15.7-21.9) |
| Restriction to studies testing all contacts at least twice | 158,10,11,12,20,23,34,39,46,54,57,59,62,73,80b | 26.2 (16.5-39.0) |
| Restriction to studies with long follow-up duration (≥21 d) | 65,8,9,23,46,92 | 32.3 (18.0-51.0) |
| Proportion of households with any secondary transmission | 157,8,9,13,17,26,30,37,46,70,72,75,84,86,92 | 35.0 (22.8-49.6) |
Abbreviation: SAR, secondary attack rate.
Restricted to studies in the US.
Restricted to studies that disaggregated SARs for at least 2 of the following: symptomatic, presymptomatic, and asymptomatic individuals.
Discussion
This updated systematic review and meta-analysis found that, with the addition of 37 studies,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40 the estimated overall household SAR of SARS-CoV-2 was 18.9%, which is similar to the estimate in the previous review.1 Nonetheless, when analyzing SAR by study period, we observed an increase in household transmission over time. Potential explanations for this temporal pattern include improved diagnostic procedures and tools, longer follow-up (which may have captured tertiary transmission or transmission from nonhousehold contacts), more contagious variants, and different study locations. We found lower SARs in studies from China and Singapore,17,84,97 potentially owing to mandated quarantine policies. It is also conceivable that the higher SARs observed may be a reflection of publication and time-trend biases, which can impact the generalizability of living systematic reviews.98
Results from the subgroup analyses reported in our previous systematic review and meta-analysis1 remained largely similar, with a few exceptions. We observed higher transmission to contacts with comorbidities across 3 studies.30,45,46 Two of these studies30,45 tested only symptomatic contacts. It is possible that testing was more common among symptomatic contacts with comorbidities.99 Individuals with comorbidities may also be more susceptible to SARS-CoV-2 infection via a number of molecular mechanisms.100 For example, Metlay et al15 reported that SARs were highest to household contacts with liver disease (25.5%), kidney disease (24.0%), and hypertension (21.6%). There was also a higher estimate of transmission from asymptomatic or presymptomatic index cases across 8 total studies6,13,14,16,24,27,58,93 compared with the transmission found in the previous meta-analysis,1 although this transmission remained considerably lower than transmission from symptomatic index cases. Studies of household transmission frequently combine these groups; however, another systematic review101 that included nonhousehold contacts reported higher transmission from presymptomatic index cases (7%; 95% CI, 3%-11%; 11 studies) than from asymptomatic index cases (1%; 95% CI, 0%-2%; 10 studies). Presymptomatic SAR is based on overall exposure before symptom onset, and presymptomatic exposure is usually of substantially shorter duration than symptomatic exposure. Most studies reporting SARs from symptomatic index cases have not separated the different phases of exposure but have combined the presymptomatic and symptomatic phases (eg, Areekal et al,4 Sundar and Bhaskar,21 and Valles et al34). This approach may partially account for lower SARs among presymptomatic index cases. Many studies included in our systematic review cautioned that they may not have identified both asymptomatic index cases and asymptomatic household contacts.
Several recent studies18,35,40,88,96,102,103,104,105,106 examined household SAR by viral variant. We limited our meta-analyses of variants to only those that were reported in 3 or more studies, which only included the B.1.1.7 (α) variant. For the B.1.1.7 (α) variant, SARs ranged from 9.0% to 42.0%35,40,96,102,103 and were reported to be higher compared with SARs for wild-type variants102 or non-VOCs104 in Ontario, Canada, and compared with SARs for other lineages in the Netherlands88 and Oslo, Norway,103 but lower compared with SARs for the B.1.617.2 (δ) variant in England.96 These findings are consistent with those reported in a modeling study105 that estimated that the transmissibility of the B.1.1.7 (α) variant was 43% to 90% higher than that of preexisting variants.
Regarding variants that were examined in fewer than 3 studies for which we did not perform meta-analyses, SARs were also higher for the B.1.351 (β) or P.1 (γ) variant (27.2%) and non-VOC variants (23.3%) compared with wild-type variants in Ontario, Canada.102Household SARs were higher for contacts with the B.1.427 and B.1.429 (ε) variants (35.6%) compared with contacts without these variants in San Francisco, California,18 whereas no major differences in household SARs were found between individuals with the B.1.526 (ι) variant and non-VOCs in New York, New York.106
Emerging data suggest that vaccination may not only be associated with the prevention of SARS-CoV-2 infections among vaccinated individuals but may also be associated with reductions in transmission to unvaccinated household contacts.29,107,108 A recent study29 (published as a preprint) of more than 1 million household contacts in England found that, compared with households in which no individuals received COVID-19 vaccines, household SARs were 40% to 50% lower among households in which index cases received BNT162b2 (Pfizer–BioNTech) or ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccines 21 days or more before receiving a positive test result for SARS-CoV-2. Another study108 (published as a preprint) of almost 200 000 household members in Scotland reported a 30% reduction in COVID-19 cases among household contacts of health care workers who received BNT162b2 or ChAdOx1 nCoV-19 vaccines at 14 days or more after the second dose compared with household contacts of health care workers who did not receive these vaccines. These findings are consistent with those of a study conducted in Finland107 that suggested indirect benefit of 8.7% (95% CI, −28.9% to 35.4%) at 2 weeks and 42.9% (95% CI, 22.3%-58.1%) at 10 weeks after the first dose of BNT162b2 or mRNA-1273 vaccines. Results suggesting a possible association between vaccination and reductions in infectiousness include lower disease severity, shorter duration of symptoms, and lower viral load.109
Limitations
This study has limitations. As described in the previous systematic review and meta-analysis,1 there was high heterogeneity across studies, which may be attributable to differences in study design (eg, follow-up duration, frequency of testing, and universal and/or symptomatic testing), transmission mitigation strategies after index case diagnosis, household crowding, underlying seroprevalence, and other factors. There was insufficient information to perform meta-analyses of SARs by other VOCs.
Conclusions
This updated systematic review and meta-analysis suggests that the household remains an important site of SARS-CoV-2 transmission, and recent studies have reported higher household SAR estimates compared with the earliest reports. More transmissible variants may be associated with further changes. Recent data suggest that 1 dose of a COVID-19 vaccine may be associated with reductions in the risk of household transmission by up to 50%,29 potentially supporting the case for universal vaccination and offering a path forward to protect household contacts.
eTable 1. Description of Studies Published From October 20, 2020, to June 17, 2021
eTable 2. Household Secondary Attack Rates for SARS-CoV-2, Restricted to Studies With a More Uniform Design
eFigure. Household Secondary Attack Rates of SARS-CoV-2 for B.1.1.7 (α) Variant
eReferences
References
- 1.Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(12):e2031756. doi: 10.1001/jamanetworkopen.2020.31756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elliott JH, Synnot A, Turner T, et al. ; Living Systematic Review Network . Living systematic review: 1: introduction—the why, what, when, and how. J Clin Epidemiol. 2017;91:23-30. doi: 10.1016/j.jclinepi.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 3.Schwarzer G, Chemaitelly H, Abu-Raddad LJ, Rucker G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods. 2019;10(3):476-483. doi: 10.1002/jrsm.1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Areekal B, Vijayan SM, Suseela MS, et al. Risk factors, epidemiological and clinical outcome of close contacts of COVID-19 cases in a tertiary hospital in southern India. J Clin Diagn Res .2021;15(3):34-37. [Google Scholar]
- 5.Awang H, Yaacob EL, Syed Aluawi SN, et al. A case-control study of determinants for COVID-19 infection based on contact tracing in Dungun district, Terengganu state of Malaysia. Infect Dis (Lond). 2021;53(3):222-225. doi: 10.1080/23744235.2020.1857829 [DOI] [PubMed] [Google Scholar]
- 6.Bender JK, Brandl M, Hohle M, Buchholz U, Zeitlmann N. Analysis of asymptomatic and presymptomatic transmission in SARS-CoV-2 outbreak, Germany, 2020. Emerg Infect Dis. 2021;27(4):1159-1163. doi: 10.3201/eid2704.204576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carazo S, Laliberté D, Villeneuve J, et al. Characterization and evolution of infection control practices among severe acute respiratory coronavirus virus 2 (SARS-CoV-2)-infected healthcare workers in acute-care hospitals and long-term care facilities in Québec, Canada, Spring 2020. Infect Control Hosp Epidemiol. 2021;1-9. doi: 10.1017/ice.2021.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerami C, Rapp T, Lin FC, et al. High household transmission of SARS-CoV-2 in the United States: living density, viral load, and disproportionate impact on communities of color. medRxiv. Preprint posted online March 12, 2021. doi: 10.1101/2021.03.10.21253173 [DOI] [PMC free article] [PubMed]
- 9.Demko ZO, Antar AAR, Blair PW, et al. Clustering of SARS-CoV-2 infections in households of patients diagnosed in the outpatient setting in Baltimore, MD. Open Forum Infect Dis .2021;8(4):ofab121. doi: 10.1093/ofid/ofab121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomaa MR, El Rifay AS, Shehata M, et al. Incidence, household transmission, and neutralizing antibody seroprevalence of coronavirus disease 2019 in Egypt: results of a community-based cohort. PLoS Pathog. 2021;17(3):e1009413. doi: 10.1371/journal.ppat.1009413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grijalva CG, Rolfes MA, Zhu Y, et al. Transmission of SARS-COV-2 infections in households—Tennessee and Wisconsin, April–September 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1631-1634. doi: 10.15585/mmwr.mm6944e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu P, Ma M, Jing Q, et al. Retrospective study identifies infection related risk factors in close contacts during COVID-19 epidemic. Int J Infect Dis. 2021;103:395-401. doi: 10.1016/j.ijid.2020.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koureas M, Speletas M, Bogogiannidou Z, et al. Transmission dynamics of SARS-CoV-2 during an outbreak in a Roma community in Thessaly, Greece—control measures and lessons learned. Int J Environ Res Public Health. 2021;18(6):2878. doi: 10.3390/ijerph18062878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li F, Li YY, Liu MJ, et al. Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: a retrospective observational study. Lancet Infect Dis. 2021;21(5):617-628. doi: 10.1016/S1473-3099(20)30981-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metlay JP, Haas JS, Soltoff AE, Armstrong KA. Household transmission of SARS-CoV-2. JAMA Netw Open. 2021;4(2):e210304. doi: 10.1001/jamanetworkopen.2021.0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyahara R, Tsuchiya N, Yasuda I, et al. Familial clusters of coronavirus disease in 10 prefectures, Japan, February-May 2020. Emerg Infect Dis. 2021;27(3):915-918. doi: 10.3201/eid2703.203882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng OT, Marimuthu K, Koh V, et al. SARS-CoV-2 seroprevalence and transmission risk factors among high-risk close contacts: a retrospective cohort study. Lancet Infect Dis. 2021;21(3):333-343. doi: 10.1016/S1473-3099(20)30833-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng J, Liu J, Mann SA, et al. ; IDseq Team . Estimation of secondary household attack rates for emergent spike L452R SARS-CoV-2 variants detected by genomic surveillance at a community-based testing site in San Francisco. Clin Infect Dis. Published online March 31, 2021. doi: 10.1093/cid/ciab283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pett J, McAleavey P, McGurnaghan P, et al. Epidemiology of COVID-19 in Northern Ireland, 26 February 2020-26 April 2020. Epidemiol Infect. 2021;149:e36. doi: 10.1017/S0950268821000224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reid MJA, Prado P, Brosnan H, et al. Assessing testing strategies and duration of quarantine in contact tracing for SARS-CoV-2: a retrospective study of San Francisco’s COVID-19 contact tracing program, June-August, 2020. Open Forum Infect Dis . Published online April 2, 2021. doi: 10.1093/ofid/ofab171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundar V, Bhaskar E. Low secondary transmission rates of SARS-CoV-2 infection among contacts of construction laborers at open air environment. Germs. 2021;11(1):128-131. doi: 10.18683/germs.2021.1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tak P, Rohilla J. COVID-19 contact tracing in a tertiary care hospital: a retrospective chart review. Infect Dis Model. 2021;6:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka ML, Marentes Ruiz CJ, Malhotra S, et al. Urban household transmission of SARS-CoV-2 during periods of high and low community transmission. SSRN. Preprint posted online March 10, 2021. doi: 10.2139/ssrn.3801730 [DOI]
- 24.Tibebu S, Brown KA, Daneman N, Paul LA, Buchan SA. Household secondary attack rate of COVID-19 by household size and index case characteristics. medRxiv. Preprint posted online February 25, 2021. doi: 10.1101/2021.02.23.21252287 [DOI]
- 25.Trunfio M, Longo BM, Alladio F, et al. On the SARS-CoV-2 “variolation hypothesis”: no association between viral load of index cases and COVID-19 severity of secondary cases. Front Microbiol. 2021;12(473):646679. doi: 10.3389/fmicb.2021.646679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkinson K, Chen X, Shaw S. Secondary attack rate of COVID-19 in household contacts in the Winnipeg health region, Canada. Can J Public Health. 2021;112(1):12-16. doi: 10.17269/s41997-020-00451-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu P, Liu F, Chang Z, et al. Assessing asymptomatic, pre-symptomatic and symptomatic transmission risk of SARS-CoV-2. Clin Infect Dis. Published online March 27, 2021. doi: 10.1093/cid/ciab271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seto J, Aoki Y, Komabayashi K, et al. Epidemiology of coronavirus disease 2019 in Yamagata Prefecture, Japan, January-May 2020: the importance of retrospective contact tracing. Jpn J Infect Dis. Published online March 31, 2021. doi: 10.7883/yoken.JJID.2020.1073 [DOI] [PubMed] [Google Scholar]
- 29.Harris RJ, Hall JA, Zaidi A, Andrews NJ, Dunbar JK, Dabrera G. Impact of vaccination on household transmission of SARS-COV-2 in England. Khub.net. Preprint posted online April 28, 2021. https://khub.net/documents/135939561/390853656/Impact+of+vaccination+on+household+transmission+of+SARS-COV-2+in+England.pdf/35bf4bb1-6ade-d3eb-a39e-9c9b25a8122a [DOI] [PMC free article] [PubMed]
- 30.Kuba Y, Shingaki A, Nidaira M, et al. The characteristics of household transmission during COVID-19 outbreak in Okinawa, Japan from February to May 2020. Jpn J Infect Dis. 2021. Published online April 30, 2021. [DOI] [PubMed] [Google Scholar]
- 31.Hsu CY, Wang JT, Huang KC, Fan ACH, Yeh YP, Chen SLS. Household transmission but without the community-acquired outbreak of COVID-19 in Taiwan. J Formos Med Assoc. 2021;120(Suppl 1):S38-S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Telle K, Jorgensen SB, Hart R, Greve-Isdahl M, Kacelnik O. Secondary attack rates of COVID-19 in Norwegian families: a nation-wide register-based study. Eur J Epidemiol. Published online May 25, 2021. doi: 10.1007/s10654-021-00760-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jashaninejad R, Doosti-Irani A, Karami M, Keramat F, Mirzaei M. Transmission of COVID-19 and its determinants among close contacts of COVID-19 patients. J Res Health Sci. 2021;21(2):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valles X, Roure S, Valerio L, et al. SARS-CoV-2 contact tracing among disadvantaged populations during epidemic intervals should be a priority strategy: results from a pilot experiment in Barcelona. Public Health. 2021;195:132-134. doi: 10.1016/j.puhe.2021.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loenenbach A, Markus I, Lehfeld AS, et al. SARS-CoV-2 variant B.1.1.7 susceptibility and infectiousness of children and adults deduced from investigations of childcare centre outbreaks, Germany, 2021. Euro Surveill. 2021;26(21):2100433. doi: 10.2807/1560-7917.ES.2021.26.21.2100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charbonnier L, Roupret-Serzec J, Caseris M, et al. Contribution of serological rapid diagnostic tests to the strategy of contact tracing in households following SARS-CoV-2 infection diagnosis in children. Front Pediatr. 2021;9(217):638502. doi: 10.3389/fped.2021.638502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verberk JDM, de Hoog MLA, Westerhof I, et al. Transmission of SARS-CoV-2 within households: a prospective cohort study in the Netherlands and Belgium—interim results. medRxiv. Preprint posted online April 26, 2021. doi: 10.1101/2021.04.23.21255846 [DOI]
- 38.Akaishi T, Kushimoto S, Katori Y, et al. COVID-19 transmission in group living environments and households. Sci Rep. 2021;11(1):11616. doi: 10.1038/s41598-021-91220-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semakula M, Niragire F, Umutoni A, et al. The secondary transmission pattern of COVID-19 based on contact tracing in Rwanda. BMJ Glob Health. 2021;6(6):e004885. doi: 10.1136/bmjgh-2020-004885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyngse FP, Molbak K, Skov RL, et al. Increased transmissibility of SARS-CoV-2 lineage B.1.1.7 by age and viral load: evidence from Danish households. medRxiv. Preprint posted online April 19, 2021. doi: 10.1101/2021.04.16.21255459 [DOI] [PMC free article] [PubMed]
- 41.Wang Z, Ma W, Zheng X, Wu G, Zhang R. Household transmission of SARS-CoV-2. J Infect. 2020;81(1):179-182. doi: 10.1016/j.jinf.2020.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Zhou Q, He Y, et al. Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. Eur Respir J. 2020;55(6):2000544. doi: 10.1183/13993003.00544-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu HJ, Hu YF, Liu XX, et al. Household infection: the predominant risk factor for close contacts of patients with COVID-19. Travel Med Infect Dis. 2020;36:101809. doi: 10.1016/j.tmaid.2020.101809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W, Zhang B, Lu J, et al. Characteristics of household transmission of COVID-19. Clin Infect Dis. 2020;71(8):1943-1946. doi: 10.1093/cid/ciaa450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez Bernal J, Panagiotopoulos N, Byers C, et al. Transmission dynamics of COVID-19 in household and community settings in the United Kingdom. medRxiv. Preprint posted online August 22, 2020. doi: 10.1101/2020.08.19.20177188 [DOI] [PMC free article] [PubMed]
- 46.Wu J, Huang Y, Tu C, et al. Household transmission of SARS-CoV-2, Zhuhai, China, 2020. Clin Infect Dis. 2020;71(16):2099-2108. doi: 10.1093/cid/ciaa557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun WW, Ling F, Pan JR, et al. Epidemiological characteristics of 2019 novel coronavirus family clustering in Zhejiang Province. Zhonghua Yu Fang Yi Xue Za Zhi .2020;54(0):E027. [DOI] [PubMed] [Google Scholar]
- 48.Bohmer MM, Buchholz U, Corman VM, et al. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: a case series. Lancet Infect Dis. 2020;20(8):920-928. doi: 10.1016/S1473-3099(20)30314-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong XC, Li JM, Bai JY, et al. Epidemiological characteristics of confirmed COVID-19 cases in Tianjin. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(5):638-641. [DOI] [PubMed] [Google Scholar]
- 50.Hua CZ, Miao ZP, Zheng JS, et al. Epidemiological features and viral shedding in children with SARS-CoV-2 infection. J Med Virol. 2020;92(11):2804-2812. doi: 10.1002/jmv.26180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y, Wang AH, Yi B, et al. Epidemiological characteristics of infection in COVID-19 close contacts in Ningbo city. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(5):667-671. [DOI] [PubMed] [Google Scholar]
- 52.Xin H, Jiang F, Xue A, et al. Risk factors associated with occurrence of COVID-19 among household persons exposed to patients with confirmed COVID-19 in Qingdao Municipal, China. Transbound Emerg Dis. 2021;68(2):782-788. doi: 10.1111/tbed.13743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Y, Song S, Kao Q, Kong Q, Sun Z, Wang B. Risk of SARS-CoV-2 infection among contacts of individuals with COVID-19 in Hangzhou, China. Public Health. 2020;185:57-59. doi: 10.1016/j.puhe.2020.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jing QL, Liu MJ, Zhang ZB, et al. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(10):1141-1150. doi: 10.1016/S1473-3099(20)30471-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang W, Cheng W, Luo L, et al. Secondary transmission of coronavirus disease from presymptomatic persons, China. Emerg Infect Dis. 2020;26(8):1924-1926. doi: 10.3201/eid2608.201142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Pan Y, Zhang D, et al. Basic epidemiological parameter values from data of real-world in mega-cities: the characteristics of COVID-19 in Beijing, China. BMC Infect Dis. 2020;20(1):526. doi: 10.1186/s12879-020-05251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park YJ, Choe YJ, Park O, et al. ; COVID-19 National Emergency Response Center, Epidemiology and Case Management Team . Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2465-2468. doi: 10.3201/eid2610.201315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park SY, Kim YM, Yi S, et al. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020;26(8):1666-1670. doi: 10.3201/eid2608.201274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu T, Liang W, Zhong H, et al. Risk factors associated with COVID-19 infection: a retrospective cohort study based on contacts tracing. Emerg Microbes Infect. 2020;9(1):1546-1553. doi: 10.1080/22221751.2020.1787799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(8):911-919. doi: 10.1016/S1473-3099(20)30287-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burke RM, Midgley CM, Dratch A, et al. Active monitoring of persons exposed to patients with confirmed COVID-19—United States, January-February 2020. MMWR Morb Mortal Wkly Rep .2020;69(9):245-246. doi: 10.15585/mmwr.mm6909e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo L, Liu D, Liao X, et al. Contact settings and risk for transmission in 3410 close contacts of patients with COVID-19 in Guangzhou, China: a prospective cohort study. Ann Intern Med. 2020;173(11):879-887. doi: 10.7326/M20-2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu S, Wang W, Wang Y, et al. Infectivity, susceptibility, and risk factors associated with SARS-CoV-2 transmission under intensive contact tracing in Hunan, China. Nat Commun. 2021;12(1):1533. doi: 10.1038/s41467-021-21710-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhuang YL, Zhang YT, Li M, et al. Analysis on the cluster epidemic of coronavirus disease 2019 in Guangdong Province. Zhonghua Yu Fang Yi Xue Za Zhi. 2020;54(7):720-725. doi: 10.3760/cma.j.cn112150-20200326-00446 [DOI] [PubMed] [Google Scholar]
- 65.Cheng HY, Jian SW, Liu DP, Ng TC, Huang WT, Lin HH; Taiwan COVID-19 Outbreak Investigation Team . Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180(9):1156-1163. doi: 10.1001/jamainternmed.2020.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim J, Choe YJ, Lee J, et al. Role of children in household transmission of COVID-19. Arch Dis Child. 2021;106(7):709-711. doi: 10.1136/archdischild-2020-319910 [DOI] [PubMed] [Google Scholar]
- 67.Zhang JZ, Zhou P, Han DB, et al. Investigation on a cluster epidemic of COVID-19 in a supermarket in Liaocheng, Shandong province. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(12):2024-2028. doi: 10.3760/cma.j.cn112338-20200228-00206 [DOI] [PubMed] [Google Scholar]
- 68.Patel A, Charani E, Ariyanayagam D, et al. New-onset anosmia and ageusia in adult patients diagnosed with SARS-CoV-2 infection. Clin Microbiol Infect. 2020;26(9):1236-1241. doi: 10.1016/j.cmi.2020.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boscolo-Rizzo P, Borsetto D, Spinato G, et al. New onset of loss of smell or taste in household contacts of home-isolated SARS-CoV-2–positive subjects. Eur Arch Otorhinolaryngol. 2020;277(9):2637-2640. doi: 10.1007/s00405-020-06066-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenberg ES, Dufort EM, Blog DS, et al. ; New York State Coronavirus 2019 Response Team . COVID-19 testing, epidemic features, hospital outcomes, and household prevalence, New York State–March 2020. Clin Infect Dis. 2020;71(8):1953-1959. doi: 10.1093/cid/ciaa549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dattner I, Goldberg Y, Katriel G, et al. The role of children in the spread of COVID-19: using household data from Bnei Brak, Israel, to estimate the relative susceptibility and infectivity of children. PLoS Comput Biol. 2021;17(2):e1008559. doi: 10.1371/journal.pcbi.1008559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewis NM, Chu VT, Ye D, et al. Household transmission of SARS-CoV-2 in the United States. Clin Infect Dis. Published online August 16, 2020. doi: 10.1093/cid/ciaa1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van der Hoek W, Backer JA, Bodewes R, et al. The role of children in the transmission of SARS-CoV-2. Ned Tijdschr Geneeskd .2020;164:D5140. [PubMed] [Google Scholar]
- 74.Dawson P, Rabold EM, Laws RL, et al. Loss of taste and smell as distinguishing symptoms of coronavirus disease 2019. Clin Infect Dis. 2021;72(4):682-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y, Tian H, Zhang L, et al. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Glob Health. 2020;5(5):e002794. doi: 10.1136/bmjgh-2020-002794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han T. Outbreak investigation: transmission of COVID-19 started from a spa facility in a local community in Korea. Epidemiol Health. 2020;42:e2020056. doi: 10.4178/epih.e2020056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bae S, Kim H, Jung TY, et al. Epidemiological characteristics of COVID-19 outbreak at fitness centers in Cheonan, Korea. J Korean Med Sci. 2020;35(31):e288. doi: 10.3346/jkms.2020.35.e288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doung-Ngern P, Suphanchaimat R, Panjagampatthana A, et al. Case-control study of use of personal protective measures and risk for SARS-CoV 2 infection, Thailand. Emerg Infect Dis .2020;26(11):2607-2616. doi: 10.3201/eid2611.203003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fateh-Moghadam P, Battisti L, Molinaro S, et al. Contact tracing during phase I of the COVID-19 pandemic in the province of Trento, Italy: key findings and recommendations. medRxiv. Preprint posted online July 29, 2020. doi: 10.1101/2020.07.16.20127357 [DOI]
- 80.Phiriyasart F, Chantutanon S, Salaeh F, et al. Outbreak investigation of coronavirus disease (COVID-19) among Islamic missionaries in southern Thailand, April 2020. Outbreak Surveill Investig Rep. 2020;13(2):48-54. [Google Scholar]
- 81.Arnedo-Pena A, Sabater-Vidal S, Meseguer-Ferrer N, et al. COVID-19 secondary attack rate and risk factors in household contacts in Castellon (Spain): preliminary report. Enfermedades Emergentes .2020;19(2):64-70. [Google Scholar]
- 82.Malheiro R, Figueiredo AL, Magalhaes JP, et al. Effectiveness of contact tracing and quarantine on reducing COVID-19 transmission: a retrospective cohort study. Public Health. 2020;189:54-59. doi: 10.1016/j.puhe.2020.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Son H, Lee H, Lee M, et al. Epidemiological characteristics of and containment measures for COVID-19 in Busan, Korea. Epidemiol Health .2020;42:e2020035. doi: 10.4178/epih.e2020035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yung CF, Kam KQ, Chong CY, et al. Household transmission of severe acute respiratory syndrome coronavirus 2 from adults to children. J Pediatr. 2020;225:249-251. doi: 10.1016/j.jpeds.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee M, Eun Y, Park K, Heo J, Son H. Follow-up investigation of asymptomatic COVID-19 cases at diagnosis in Busan, Korea. Epidemiol Health. 2020;42:e2020046. doi: 10.4178/epih.e2020046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Draper AD, Dempsey KE, Boyd RH, et al. The first 2 months of COVID-19 contact tracing in the northern territory of Australia, March-April 2020. Commun Dis Intell (2018). 2020;44:1-10. doi: 10.33321/cdi.2020.44.53 [DOI] [PubMed] [Google Scholar]
- 87.Teherani MF, Kao CM, Camacho-Gonzalez A, et al. Burden of illness in households with severe acute respiratory syndrome coronavirus 2–infected children. J Pediatric Infect Dis Soc. 2020;9(5):613-616. doi: 10.1093/jpids/piaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lyngse FP, Kirkeby CT, Halasa T, et al. COVID-19 transmission within Danish households: a nationwide study from lockdown to reopening. medRxiv. Preprint posted online September 9, 2020. doi: 10.1101/2020.09.09.20191239 [DOI]
- 89.Islam SS, Noman ASM. Transmission dynamics and contact tracing assessment of COVID-19 in Chattogram, Bangladesh and potential risk of close contacts at different exposure settings. SSRN. Preprint posted online October 12, 2020. doi: 10.2139/ssrn.3677863 [DOI]
- 90.Adamik B, Bawiec M, Bezborodov V, et al. ; MOCOS International Research Group. Bounds on the total number of SARS-CoV-2 infections: the link between severeness rate, household attack rate and the number of undetected cases. ResearchGate. Preprint posted online July 29, 2020. doi: 10.13140/RG.2.2.30750.77124 [DOI]
- 91.Laxminarayan R, Wahl B, Dudala SR, et al. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science. 2020;370(6517):691-697. doi: 10.1126/science.abd7672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shah K, Desai N, Saxena D, Mavalankar D, Mishra U, Patel GC. Household secondary attack rate in Gandhinagar district of Gujarat state from western India. medRxiv. Preprint posted online September 5, 2020. doi: 10.1101/2020.09.03.20187336 [DOI]
- 93.Chaw L, Koh WC, Jamaludin SA, Naing L, Alikhan MF, Wong J. Analysis of SARS-CoV-2 transmission in different settings, Brunei. Emerg Infect Dis. 2020;26(11):2598-2606. doi: 10.3201/eid2611.202263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.COVID-19 National Emergency Response Center; Epidemiology Case Management Team; Korea Centers for Disease Control and Prevention. Coronavirus disease–19: summary of 2,370 contact investigations of the first 30 cases in the Republic of Korea. Osong Public Health Res Perspect. 2020;11(2):81-84. doi: 10.24171/j.phrp.2020.11.2.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu R, Han H, Liu F, et al. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta. 2020;505:172-175. doi: 10.1016/j.cca.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Public Health England . SARS-CoV-2 variants of concern and variants under investigation in England. Public Health England; 2021. Technical briefing 14. Accessed June 10, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/991343/Variants_of_Concern_VOC_Technical_Briefing_14.pdf
- 97.Hou C, Chen J, Zhou Y, et al. The effectiveness of quarantine of Wuhan city against the corona virus disease 2019 (COVID-19): a well-mixed SEIR model analysis. J Med Virol. 2020;92(7):841-848. doi: 10.1002/jmv.25827 [DOI] [PubMed] [Google Scholar]
- 98.Elvik R. Publication bias and time-trend bias in meta-analysis of bicycle helmet efficacy: a re-analysis of Attewell, Glase and McFadden, 2001. Accid Anal Prev. 2011;43(3):1245-1251. doi: 10.1016/j.aap.2011.01.007 [DOI] [PubMed] [Google Scholar]
- 99.Apra C, Caucheteux C, Mensch A, et al. ; AP-HP/Universities/Inserm COVID-19 Research Collaboration. Predictive usefulness of PCR testing in different patterns of Covid-19 symptomatology—analysis of a French cohort of 12,810 outpatients. medRxiv. Preprint posted online June 9, 2020. doi: 10.1101/2020.06.07.20124438 [DOI]
- 100.Alyammahi SK, Abdin SM, Alhamad DW, Elgendy SM, Altell AT, Omar HA. The dynamic association between COVID-19 and chronic disorders: an updated insight into prevalence, mechanisms and therapeutic modalities. Infect Genet Evol. 2021;87:104647. doi: 10.1016/j.meegid.2020.104647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qiu X, Nergiz AI, Maraolo AE, Bogoch II, Low N, Cevik M. The role of asymptomatic and pre-symptomatic infection in SARS-CoV-2 transmission-a living systematic review. Clin Microbiol Infect. 2021;27(4):511-519. doi: 10.1016/j.cmi.2021.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brown KA, Tibebu S, Daneman N, Schwartz K, Whelan M, Buchan S. Comparative household secondary attack rates associated with B.1.1.7, B.1.351, and P.1 SARS-CoV-2 variants. medRxiv. Preprint posted online June 4, 2021. doi: 10.1101/2021.06.03.21258302 [DOI]
- 103.Lindstrom JC, Engebretsen S, Brathen Kristoffersen A, et al. Increased transmissibility of the B.1.1.7 SARS-CoV-2 variant: evidence from contact tracing data in Oslo, January to February 2021. medRxiv. Preprint posted online March 30, 2021. doi: 10.1101/2021.03.29.21254122 [DOI] [PubMed]
- 104.Buchan SA, Tibebu S, Daneman N, et al. Increased household secondary attacks rates with variant of concern SARS-CoV-2 index cases. Clin Infect Dis. Published online June 9, 2021. doi: 10.1093/cid/ciab496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davies NG, Abbott S, Barnard RC, et al. ; CMMID COVID-19 Working Group; COVID-19 Genomics UK (COG-UK) Consortium . Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372(6538):eabg3055. doi: 10.1126/science.abg3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thompson CN, Hughes S, Ngai S, et al. Rapid emergence and epidemiologic characteristics of the SARS-CoV-2 B.1.526 variant—New York City, New York, January 1-April 5, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(19):712-716. doi: 10.15585/mmwr.mm7019e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salo J, Hagg M, Kortelainen M, et al. The indirect effect of mRNA-based Covid-19 vaccination on unvaccinated household members. medRxiv. Preprint posted online May 29, 2021. doi: 10.1101/2021.05.27.21257896 [DOI] [PMC free article] [PubMed]
- 108.Shah ASV, Gribben C, Bishop J, et al. Effect of vaccination on transmission of COVID-19: an observational study in healthcare workers and their households. medRxiv. Preprint posted online March 21, 2021. doi: 10.1101/2021.03.11.21253275 [DOI]
- 109.Richterman A, Meyerowitz EA, Cevik M. Indirect protection by reducing transmission: ending the pandemic with SARS-CoV-2 vaccination. Open Forum Infect Dis . Published online May 19, 2021. doi: 10.1093/ofid/ofab259 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Description of Studies Published From October 20, 2020, to June 17, 2021
eTable 2. Household Secondary Attack Rates for SARS-CoV-2, Restricted to Studies With a More Uniform Design
eFigure. Household Secondary Attack Rates of SARS-CoV-2 for B.1.1.7 (α) Variant
eReferences