Abstract
Cotton is a potential and excellent candidate to balance both agricultural production and remediation of mercury-contained soil, as its main production fiber hardly involves into food chains. However, in cotton, there is known rarely about the tolerance and response to mercury (Hg) environments. In this study, the biochemical and physiological damages, in response to Hg concentrations (0, 1, 10, 50 and 100 µM), were investigated in upland cotton seedlings. The results on germination of cottonseeds indicated the germination rates were suppressed by high Hg levels, as the decrease of percentage was more than 10% at 1000 µM Hg. Shoots and roots’ growth were significantly inhibited over 10 µM Hg. The inhibitor rates (IR) in fresh weight were close in values between shoots and roots, whereas those in dry weight the root growth were more obviously influenced by Hg. In comparison of organs, the growth inhibition ranked as root > leaf > stem. The declining of translocation factor (TF) opposed the Hg level as even low to 0.05 at 50 µM Hg. The assimilation in terms of photosynthesis, of cotton plants, was affected negatively by Hg, as evidenced from the performances on pigments (chlorophyll a and b) and gas exchange (Intercellular CO2 concentration (Ci), CO2 assimilation rate (Pn) and stomatal conductance (Gs)). Sick phenotypes on leaf surface included small white zone, shrinking and necrosis. Membrane lipid peroxidation and leakage were Hg dose-dependent as indicated by malondialdehyde (MDA) content and relative conductivity (RC) values in leaves and roots. More than 10 µM Hg damaged antioxidant enzyme system in both leaves and roots (p < 0.05). Concludingly, 10 µM Hg post negative consequences to upland cotton plants in growth, physiology and biochemistry, whereas high phytotoxicity and damage appeared at more than 50 µM Hg concentration.
Keywords: upland cotton (Gossypium hirsutum L.), mercury stress, phytotoxicity, physiological and biochemical response
1. Introduction
Heavy metal (HM) pollution, as a global problem affecting terrestrial and aquatic environments, also posts a potential threat to human health via the food chains. Meanwhile, excessive HM concentrations in soils resulted in yield reduction and poor quality of crop products [1]. Mercury (Hg) is one of the most toxic HM because of its easy bioaccumulation in living bodies [2]. Hg may enter agricultural soil through various anthropogenic activities including fertilizers, pesticides, sludge, lime and manure. It exists in nature in forms like elemental mercury, ionic mercury, methyl mercury, mercury sulfide and mercury hydroxide. In all sorts of mercury, ionic mercury is the predominant toxic form [3,4]. In plant, the toxic action of Hg is first done on the roots, which take up Hg directly in an efficient manner [5]. While entering the root cells, Hg may bind to the water channel proteins of root cells and thus causes physical obstruction to water flowing, consequently affecting the transpiration in plants [6,7]. Furthermore, it has also been reported that Hg suppresses photosynthesis, chlorophyll synthesis, as well as uptake and transport of nutrients. Hg is considered to inhabit the activity of the NADPH: protochlorophyllide oxidoreductase (POR), which plays key roles in photosynthesis, consequently affects plant growth involving biomass [8]. Sahu evaluated the oxidative damages response to Hg concentrations in wheat [9], representing lower and higher Hg concentration induced and repressed antioxidant enzymes activities separately, which supported the opinion that Hg can boost the formation of reactive oxygen species (ROS) and consequently pose more serious oxidative stress on plant cell.
Cottons (Gossypiums) are the most important source of natural fiber as well as key sources of edible oil in the world. Due to its hard wooden nature, cotton presents relatively multiple resistances to abiotic stress [10]. Previous works regarding HM stresses on cotton revealed varied behavior of cotton cultivars towards various toxic HM, like cadmium, chromium, copper and lead. Under sorts of HM with concentrations, there appeared anomalies in cotton plants. The performance could be detected, such as a reduction in germination at higher levels of metals, a decline in the biometric parameters like root and shoot length, or fresh and dry biomass, as well as altering on contents of biochemical indictors [11,12,13,14,15]. Regarding the cotton behavior towards Hg, no such study has so far been conducted. Keeping in view of the global usage of Hg in various industries, the present experiment was designed with the aim to investigate the toxic effects of Hg on upland cotton by studying various physiological and biochemical parameters.
2. Results
2.1. Effects of Hg on Cotton Seeds Germination
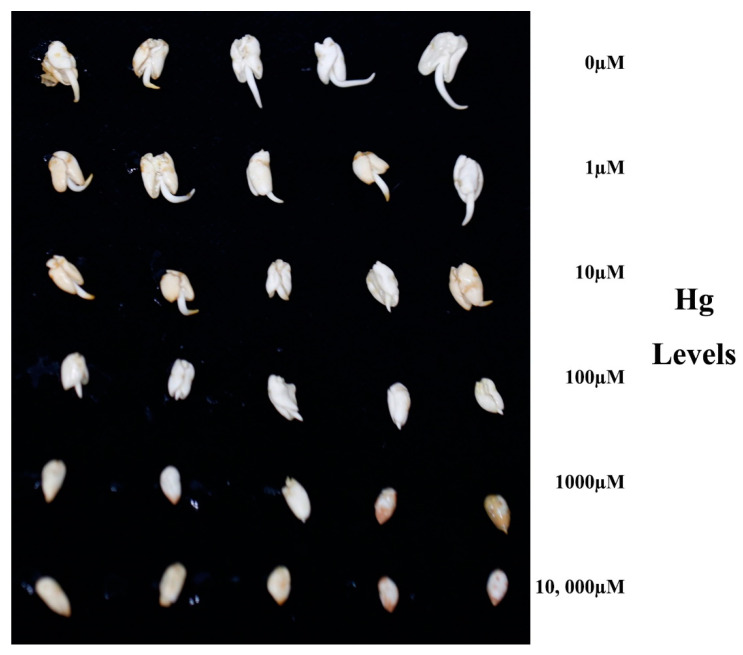
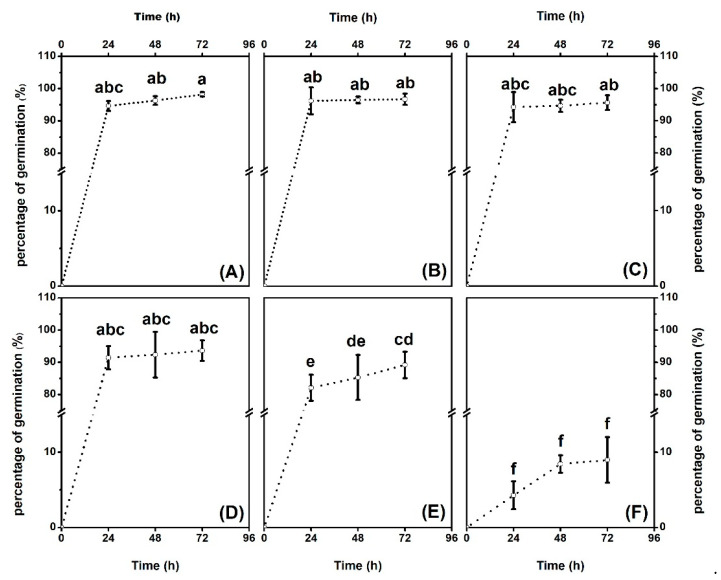
In the present study, a Hg concentration ranging from 0 to 10,000 µM was employed in order to test the performances on cottonseed germination. Elongation of seed shoot and color diversity, paralleling with the percentage of germination, were studied. Changes in morphology and percentage of seed germination were obvious and presented as Figure 1 and Figure 2. Overall, higher Hg concentrations treatment depressed seed shoot elongation, darkened the seeds surface and reduced percentage of germination, comparing with low levels. In details, 1 and 10 µM Hg did not affect seed shoot elongation, whereas significances could be observed in the doses 100, 1000 and 10,000 µM. The seed surface color was white in both the control and treatment of 100 µM Hg. However, in the groups of 1000 and 10,000 µM, the seed surface turned brown, which was considered as sick. The percentage of germination was tested within a course of 72 h. As revealed in Figure 2, there were no significant variations (p < 0.05) in germination percentage from the control to 100 µM, and they maintained percentages above 90% for 72 h. However, the germination percentage decreased starting at 1000 µM Hg, and dropped to 80%~90%. At 10,000 µM Hg, the germination percentage decreased sharply to less than 10%.
Figure 1.
Morphology on germination of decoated seeds TM-1 under Hg treatments. Decoated seeds exposed to Hg2+ solution with gradient 0, 1, 10, 100, 1000 and 10,000 µM, which were presented from upper to lower rows respectively.
Figure 2.
Germination rate of decoated cotton seeds under Hg concentrations during a course of 72 h. (A–F): De-coated cotton seeds exposed to Hg2+ solution with concentration at 0, 1, 10, 100, 1000 and 10,000 μM, respectively, and the germination experiment was conducted under shaking condition at 130 rpm and 28 °C in dark. The percentages of germination were determined at 0, 24, 48 and 72 h. Error bars represent SD value (n = 3). Different letters indicate significant differences (p < 0.05).
2.2. Growth and Hg Distribution
The weight of cotton seedlings declined after HgCl2 treatment for a week. The reduction in both shoots and roots in fresh and dry weight was dose-dependent (Table 1). In shoots, there was no significant difference in fresh weight between the control and 1 µM Hg (p < 0.05). At 10 µM Hg, the mean value of shoots, in terms of fresh weight, was 65.55 g, as the inhibitory rate (IR) was 18.8%. When the concentration was over 50 µM, the IR seemed to approach the summit. In details, the values were 36.26% and 37.32% at 50 and 100 µM Hg, separately, which were quite close each other. Regarding the dry weight in shoots, it can be affected by elevated Hg concentrations and exhibited analogous trends with that in fresh weight, even though there was no statistical difference at 10 µM Hg. Correspondingly, the growth inhibition of both fresh and dry weight in roots was in a dose-dependent manner with increased Hg level. What is more, the significant IR change alternativeness (comparing with the controls) on fresh and dry weight of roots began at 10 µM Hg, and the IR value was over 32.10 at 50 and 100 µM Hg. Overall, the cotton seedlings treated by 10 µM Hg for a week will show significant growth inhibition and inhibitory upper limit appeared at approximately 50 µM Hg. Comparing to shoots as well as fresh weight separately, the growth of relevant roots and dry weight was more easily suppressed by Hg toxicity.
Table 1.
Effects of Hg2+ levels on growth in shoots and roots.
| Hg2+ Treatment (μM/L) | Shoots | Roots | ||||||
|---|---|---|---|---|---|---|---|---|
| Fresh Weight (g) |
IR (FW) (%) |
Dry Weight (g) |
IR (DW) (%) |
Fresh Weight (g) |
IR (FW) (%) |
Dry Weight (g) |
IR (DW) (%) |
|
| 0 | 80.73 ± 1.22 a | 0 | 6.76 ± 0.69 a | 0 | 21.62 ± 0.06 a | 0 | 1.91 ± 0.28 a | 0 |
| 1 | 79.89 ± 1.97 a | 1.04 | 6.54 ± 0.44 a | 3.25 | 20.78 ± 1.03 a | 3.89 | 1.81 ± 0.10 a | 5.24 |
| 10 | 65.55 ± 5.37 b | 18.8 | 5.95 ± 0.69 ab | 11.98 | 17.58 ± 1.36 b | 18.69 | 1.51 ± 0.13 b | 20.94 |
| 50 | 51.42 ± 3.23 c | 36.26 | 5.42 ± 0.11 b | 19.82 | 14.68 ± 1.90 c | 32.10 | 1.07 ± 0.15 c | 43.98 |
| 100 | 50.58 ± 4.14 c | 37.32 | 5.40 ± 0.10 b | 20.19 | 13.91 ± 0.69 c | 36.16 | 0.94 ± 0.07 c | 49.21 |
Error bars represent SD value (n = 3) and different letters indicate significant differences (p < 0.05).
Hg was accumulated in all organs involved in leaves, stems and roots after the treatments (Table 2). The roots concentrated most Hg, followed by leaves and stems. The concentration index ranged from 1 to 58.89, 1 to 7.22 and 1 to 4.02 in those 3 sorts of organs, respectively and orderly. As a whole on means data, Hg enrichment in roots was 8- and 16-fold that in leaves and stems separately. With the increased Hg concentrations, the translocation factor (TF) in cotton seedlings decreased. At 50 and 100 µM Hg, the TF value declined to 0.05 and 0.06, respectively, which represented the limit of Hg translocation.
Table 2.
Mercury distributions in tissues and their concentration and translocation in seedlings.
| Hg2+ Treatments (μM) | Hg Concentration (μgg−1 DW) | CI (Concentration Index) | TF (Translocation Factor) | ||||
|---|---|---|---|---|---|---|---|
| Leaves | Stems | Root | Leaves | Stems | Roots | ||
| 0 | 1.45 ± 0.10 a | 1.63 ± 0.28 a | 5.16 ± 0.05 a | 1.00 | 1.00 | 1.00 | 0.60 |
| 1 | 2.23 ± 0.12 b | 2.28 ± 0.19 b | 28.60 ± 0.36 b | 1.54 | 1.40 | 5.54 | 0.16 |
| 10 | 3.86 ± 0.10 b | 2.42 ± 0.01 c | 54.81 ± 0.33 c | 2.66 | 1.48 | 10.62 | 0.11 |
| 50 | 9.37 ± 0.45 c | 4.05 ± 0.06 d | 288.23 ± 20.75 d | 6.46 | 2.48 | 55.86 | 0.05 |
| 100 | 10.47 ± 0.27 d | 6.56 ± 0.01 e | 303.89 ± 11.58 d | 7.22 | 4.02 | 58.89 | 0.06 |
Error bars represent SD value (n = 3) and different letters in same group indicate significant differences (p < 0.05). μgg−1 DW indicates the amount in terms of microgram Hg per gram in dry tissue sample.
2.3. Effects of Hg on Cotton Assimilation
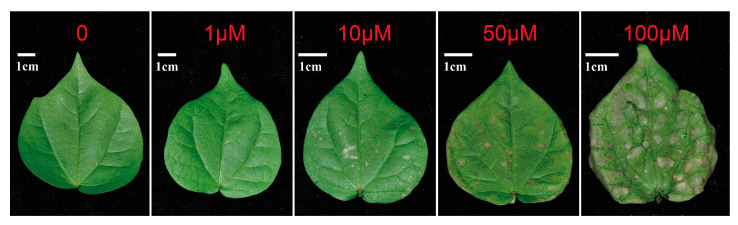
The growth of treated plants was inhibited under Hg stress, which may indicate the decline on assimilations regarding photosynthesis. The phenotypes on leaf surface, as well as pigments and gas exchange involved in photosynthesis, were studied. Comparingly, much severe sick phenotypes appeared on leaves under higher Hg concentration (Figure 3). At 10 µM Hg, small white zone emerged on the leaf upper surface, whereas obvious necrosis and shrinking happened at 50 and 100 µM Hg. Obviously, the extent of leaf health inversely synchronized to the IR depending on Hg levels.
Figure 3.
Morphological leaves responding to Hg levels at 0, 1, 10, 50 and 100 µM. The scale bars denote 1 cm.
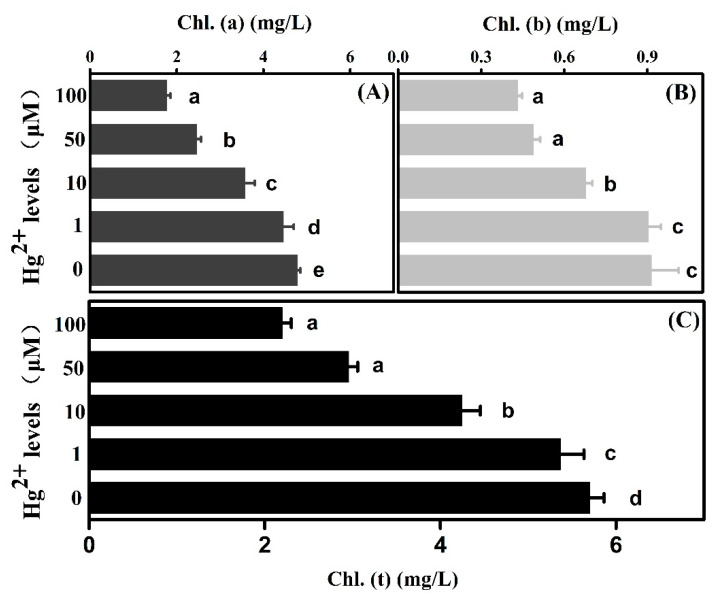
The average amounts of photosynthesis pigments, chlorophyll a and b, showed a similar decreased trend exposure to more severe Hg pollution (Figure 4). Chlorophyll a decreased to 93.2%, 74.6%, 51.5% and 36.9% at 1, 10, 50 and 100 μM Hg concentration, respectively, in comparison with the control. Meanwhile, 98.8%, 74.0%, 53.5% and 47.2% declines were found in chlorophyll b as compared with the control. The total chlorophyll content, comprised of chlorophyll a plus b, exhibited the declining trends. In comparison to the control, the total chlorophyll reduced to 94.1%, 74.5%, 51.8% and 38.5% at Hg treatments of 1, 10, 50 and 100 µM, respectively.
Figure 4.
Pigment content in leaves under stress from Hg levels. (A–C): chlorophyll a, chlorophyll b and total chlorophyll contents, as abbreviated to Chl. (a), Chl. (b) and Chl. (t), are shown, respectively. Error bars represent SD value (n = 3). Different letters indicate significant differences (p < 0.05).
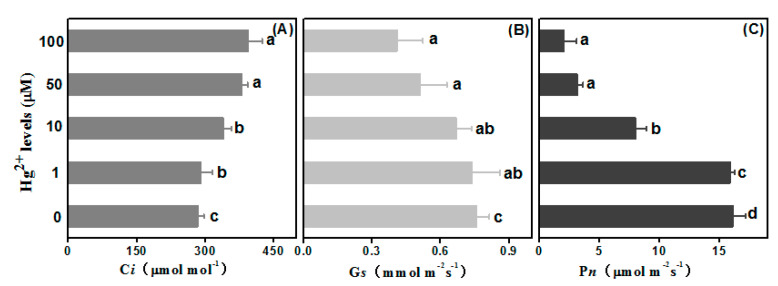
Determination on leaf gas exchange parameters must help to track photosynthesis efficiency influenced by Hg stress (Figure 5). Compared with the control value 283.54 µmol·mol−1, Ci was increased to 290.34, 340.15, 380.31 and 395.21 µmol·mol−1 at 1, 10, 50 and 100 µM Hg treatments, correspondingly. A significant reduction in Gs was noted at 50 and 100 µM Hg treatments, and those were decreased to 67.11% and 53.95% in comparison to the control. On the contrary, Gs value in 1 and 10 μM Hg treatments did not present a significant increase compared to the control (Figure 5B). Concerning Pn, there was no significant difference between 1 µM Hg and the control. However, profound value gaps on Pn were shown at 10, 50 and 100 μM Hg, as reduced to 49.75%, 19.54% and 12.59%, separately.
Figure 5.
Gas exchange in leaves under stress from Hg levels. The column (A–C) are exhibited as the intercellular CO2 concentration (Ci), stomatal conductance (Gs) and CO2 assimilation rate (Pn), respectively. Error bars represent SD value (n = 3). Different letters indicate significant differences (p < 0.05).
2.4. Membrane Lipid Peroxidation and Leakage
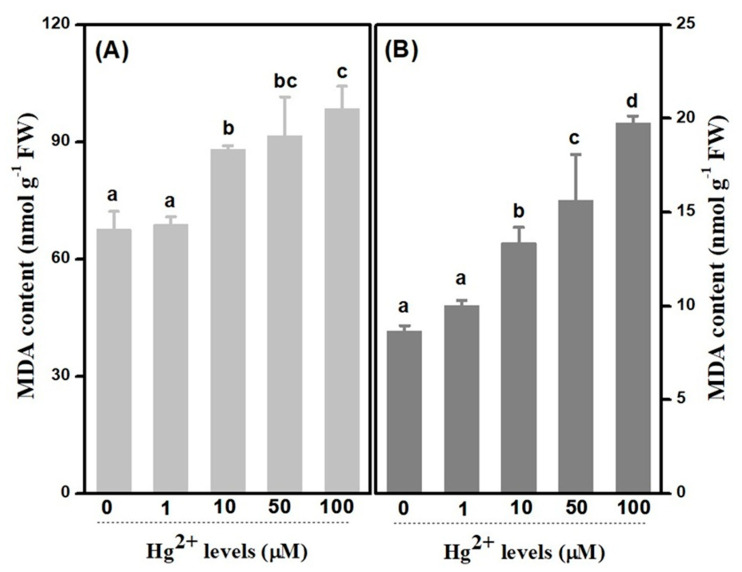
Accumulation of malondialdehyde (MDA), as a key indicator of membrane lipid peroxidation, is commonly employed to evaluate cell injury under stresses [16]. MDA has been induced in both leaves and roots with the raising of treated Hg concentrations. As a whole, MDA accumulations in leaves were estimated 6-fold on average as much as that in roots (Figure 6). In leaves, amounts of MDA were increased apparently to 1.30, 1.35 and 1.46 times at 10, 50 and 100 µM Hg treatments, respectively, in comparison with the control, whereas those increased to 1.54, 1.80 and 2.28 times in roots, correspondingly.
Figure 6.
MDA content under Hg levels in leaves and roots. (A): MDA content under Hg2+ levels in leaves; (B): MDA content under Hg2+ levels in roots. Error bars represent SD value (n = 3) and different letters indicate significant differences (p < 0.05).
Effects of variable Hg stresses on electrolyte leakage (EL), in both leaves and roots, were investigated via relative conductivity (RC) (Table 3). Hg stress induced increase of EL significantly. In leaves, the RC value in the control was 15.54%, and that was 15.85%, 30.30%, 53.95% and 78.36% at 1, 10, 50 and 100 μM Hg, respectively. In roots, correspondingly, RC was 35.22%, 38.64%, 52.10% and 54.01% at Hg treatments, which were significantly much more than that in the control as value 20.21%.
Table 3.
Effects of Hg2+ levels on relative conductivity (RC) in leaves and roots.
|
Hg2+ Levels (μM) |
Relative Conductivity (RC) (%) | |
|---|---|---|
| Leaves | Roots | |
| 0 | 15.54 ± 1.83 a | 20.21 ± 3.53 a |
| 1 | 15.85 ± 2.83 a | 35.22 ± 3.59 b |
| 10 | 30.30 ± 7.81 b | 38.64 ± 0.87 b |
| 50 | 53.95 ± 5.07 c | 52.10 ± 7.73 c |
| 100 | 78.36 ± 8.68 d | 54.01 ± 5.97 c |
Error bars represent SD value (n = 3) and different letters indicate significant differences (p < 0.05).
2.5. Antioxidant Enzyme Systems Response
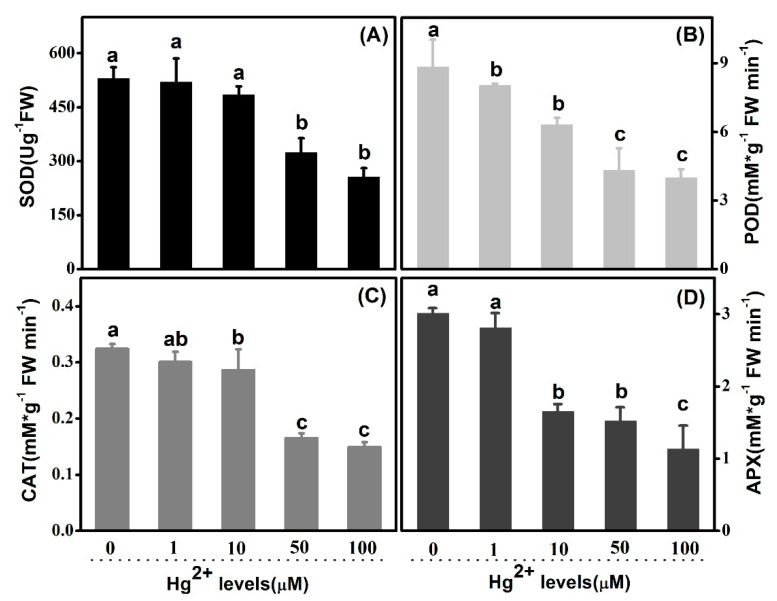
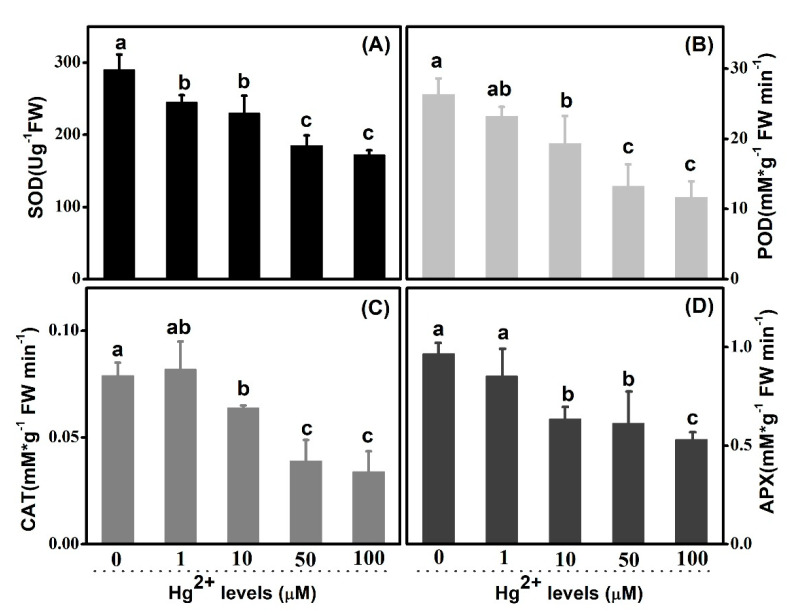
Antioxidant enzymes were analyzed in both roots and leaves, in order to study their response in scavenging of ROS during the course of external application of Hg. SOD activities, both in leaves and roots, were inhibited gradually with the rising of Hg concentrations. Wholly, the SOD activities of leaves were higher than that of roots (Figure 7A and Figure 8A). In leaves and comparing with the control, there was no significant decrease at 1 and 10 µM Hg. However, the great decrease of values appeared at both 50 and 100 µM. However, SOD activities of treated plants went down to 84.5%, 79.3%, 63.8% and 59.3%, correspondingly, relative to the untreated control in roots. Concerning POD, similar trends were shown in both leaves and roots as SOD activity. POD activities were apparently lower in leaves than those in roots wholly. In comparison to the control value 8.836 mM g−1 FW min−1, POD activities were decreased to 8.031, 6.324, 4.324 and 3.986 mM g−1 FW min−1 at 1, 10, 50 and 100 µM Hg in leaves, respectively (Figure 7B). Correspondingly, in roots, those values were 26.350, 23.240, 19.340, 13.250 and 11.648 mM g−1 FW min−1 from 0 to 100 µM Hg treatment (Figure 8B). As regards CAT, the activities were suppressed profoundly by high-level Hg treatments in both leaves and roots (Figure 7C and Figure 8C). CAT activities in leaves decreased to 50.93% and 45.99% at 50 and 100 µM Hg as compared with control, and those decreased to 49.37% and 43.04% in roots, separately. The activity was 0.082 mMg−1 FW min−1 at 1 µM Hg in roots, which was higher than the control 0.079 mMg−1 FW min−1, although there was no statistical difference. As shown in Figure 7D and Figure 8D, APX activities were inhibited significantly at 10, 50 and 100 µM Hg, in both leaves and roots. Furthermore, the activity bottom appeared at concentration of 100 µM Hg, as 1.135 and 0.532 mM g−1 FW min−1 in leaves and roots, separately. However, there was no significance between the control and 1 µM level.
Figure 7.
Effects of Hg on SOD, POD, CAT and APX activity in functional leaves. (A–D): SOD, POD, CAT and APX activity is shown successively and respectively. Error bars represent SD value (n = 3). Different letters indicate significant differences (p < 0.05).
Figure 8.
Effects of Hg on SOD, POD, CAT and APX activity in roots. (A–D): SOD, POD, CAT and APX activity is shown successively and respectively. Error bars represent SD value (n = 3). Different letters indicate significant differences (p < 0.05).
3. Discussion
Mercury (Hg), as a highly toxic nonessential element and its dispersion in the environment, is considered as a serious environmental problem for its persistent character [17]. Unlike most metals that function as nutrients, Hg has no known physiological action, so it is not metabolized by most organisms [2]. Knowledges in terms of the toxic effects of Hg in plants may be conducive to the general understanding on the primary toxicity mechanism and the tolerant characters in living organisms such as upland cotton.
3.1. Seeds Versus Seedling Plants Exhibited Higher Tolerance to Hg Toxicity
Seed germination and seedling growth are convenient and simple end points to be employed for evaluating the toxicity of pollutants to higher plants at the early stages, which are associated with biomass directly [18]. In the present study, a wide range of Hg concentration treatments from 0 to 10,000 µM was chosen for detection of inhibition on germination. The results on germination indicated that cottonseed was able to bear extra-high Hg concentration stress. Seed GR maintained over 80%, although the Hg concentration was as much as 1000 µM. Significant differences were easily detected on the fresh or dry weight of both leaves and roots separately even in the 10 µM treated plants, compared to the relevant controls. As a consequence, cotton seeds exhibited much more tolerance to Hg stress, in comparison with the seedling plants. In addition, 50 µM Hg stress never affected the seed germination of Quercus ilex, but the root fresh weight and longest root length were easily depressed even by 5 μM Hg [19]. Ling reported that seed germination was not repressed at 100 µM Hg concentration in four kinds of vegetables including cabbage (Brassica rapa), cole (B. napus), head cabbage (B. oleracea) and spinach (Spinacia oleracea) [20]. Additionally, the wheat seed GR decreased by approximately merely 10% under the Hg concentration as high as 1000 μM [21]. In present experiments in seedlings, their growth seemed to be inhibited more than 10 µM Hg. It is obvious that Hg2+ are likely to be aborted by cotton root and subsequently transported to the whole plant. Our results are consistent with documented species. It is clear that either woody or herbal plants showed relatively more tolerance and sensitivity to Hg environment in seeds and plants, respectively. Seeds, as the key energy bank to organism survival, store amounts of nutrition and process stronger vigor comparing with other organs, probably conferring the ability to face the change on the environment involving HM. Roots are the primary organ to contact the Hg, which could be distributed to the overall plant via the phloem. Phytotoxicity of Hg resulted from loss of function on targeted aquaporin [22,23]; thus, this protein family in cotton is sensitive to Hg. In wheat, significant phytotoxicity was at above 10 μM Hg concentration, as the phytotoxicity appeared in upland cotton. Thus, cotton seedling exhibited some native tolerance to Hg toxicity, whereas it seemed not too apparent the advantages to other main field crops [9]. Further and comprehensive studies need to be conducted in order to reveal the spatial and temporal responses of Hg on cotton plants, and those would enlighten the roads to remediate Hg-containing soil via cotton.
3.2. Damage of Photosynthesis Resulting from Hg Toxicity Accounted for the Sick Growth
Plant growth is the function in terms of cell wall extensibility, osmotic potential, water conductivity, and threshold turgor, among other factors. Growth does not occur when these agents are insufficient [24]. Decrease in seedling growth involving biomass has been well documented in plants under Hg stress. This might be attributed to the lack of essential elements such as Fe, S and Zn, and decreasing photosynthesis as Hg poison [8]. As inhibition on growth in response to Hg toxicity in upland cotton, a similar response has also been detected in soybean (Glycine max), radish (Raphanussativus), tomato (Solanum lycopersicum) and other plants [25,26]. Hg also damaged photosynthetic pigments, which represented a declining of contents of chlorophyll. It was reported that HM depresses chlorophyll formation via interfering with protochlorophyllide production [27]. HM might be also replaced by the Mg from chlorophyll molecules in green plants [28], and thereby chlorophyll pigments reduce the photosynthetic efficiency undoubtedly.
The leaf gas exchange study indicated that photosynthetic parameters, referring to Gs, Pn and Ci, were associated with the inhibition of growth due to Hg stress. Those were reported in plants such as white lupin (Lupinus albus L.), rice (Oryza sativa L.) and Arabidopsis (Arabidopsis thaliana) [29,30]. In addition, these agree with our results that a rise in intercellular CO2 concentration and a decline in both stomatal conductance and CO2 assimilation rate are due to increasing Hg levels. Thus, reduction of biomass under Hg stress may be the consequence of reduction in photosynthesis pigments and following blocking of photosynthesis. Declined Pn may result from the inhibition of relevant reaction steps in the Benson–Calvin cycle and rubisco activity influenced by these contaminants [31]. Moreover, a decrease in Pn could be due to stomatal closing.
3.3. Decrease on inTegrity of Membrane-Symbolled Cellular Injure Resulting from Hg Toxicity
Being analogous to other abiotic stress, HMs damage membrane is largely mediated via membrane lipid peroxidation, leading to EL [16]. It is reported that an increase in MDA contents in response to Hg stress has been detected, both in leaves and roots, from Medicageo sativa and other plants [32]. Significant accumulations of MDA content were observed, both in leaves and roots, from treatments which were more than 10 µM Hg concentration, in upland cotton plants. These results imply that the membranes are injured in consequence of reactive oxygen species, as cotton seedlings are contaminated by high Hg levels. Rising of percentage in regard to electrolytic leakage has been observed both in the leaves and roots of upland cotton seedling treated with higher than 10 µM Hg concentration as well. It is concluded that root, rather than leaf, is more sensitive to Hg stress, resulting from the fact that significant variances were detected in 1 µM Hg level in roots but not in leaves, as compared with the controls, respectively, which is in agreement with the result of SOD. The sensitivity to Hg stress was relatively serious in roots as compared to leaves, which may be attributed to Hg accumulation in roots being faster as they are exposed to Hg iron directly. Lipid peroxidation resulting from Hg stress has been reported in many plants, and Mishra have found an increase both of MDA and electrical conductivity in Oryza sativa by treated with Hg concentration, which is in line with our results in upland cotton [33].
3.4. Hg Disturbed Cellular Redox Equilibrium Involving Depression on Activities of Antioxidant Enzyme System
As a transition element, oxidative stress can be induced by Hg in consequence of excess production of reactive oxygen species (ROS) in plants, leading to alternative antioxidant enzymes activities, lipid peroxidation and ion leakage [34]. Plants, with a set of potential mechanisms, might be associated with metal detoxification at the cellular level, and tolerance in plant is shown in HMs. The generation of ROS induced by Hg triggers influences activation of components in the antioxidative defense system in plants, as it consists of both the enzymatic system, which includes SOD, POD, APX, CAT and others, and the non-enzymatic system, containing some low-molecular-weight antioxidants such as glutathione and ascorbic acid, etc. Overall, exposure to higher concentrations of Hg concerning 50 and 100 µM altered the activities of all four sorts of antioxidant enzymes, both in leaves and root, in which Hg ions at low level (1µM) did not lead to significant alternation, although, as compared with the controls, respectively. In conclusion, relevant activities of antioxidant enzymes are suppressed just under higher Hg concentration, rather than lower Hg treatment. The enzymatic antioxidant SOD, as the first enzyme in the detoxifying process, is considered an essential component among antioxidant defense systems in plants [35,36]. Root is more sensitive to Hg than leaf, resulting from the fact that more significant variances, in terms of SOD, were detected in roots rather than in leaves in lower Hg levels, including 1 and 10 μM. The changes in activity of POD have been observed under serious Hg levels. The trend in leaves is in line with that in roots, thus it is hypothesized that POD activity might be influenced in leaf and root approximately. Regarding CAT, the activity was inhabited by Hg levels both in leaves and roots overall, while that was slightly enhanced in 1 µM Hg concentration in roots. It shows that CAT activity could be triggered by the lower concentration in roots to some extent. It has been well documented that some sorts of heave metal with low concentration stimulated activities of antioxidant enzymes, differing from the sort of the enzymes, Hg concentration levels and plants species [9,37,38,39]. In response to Hg stress, activity of APX exhibited parallel trends with that of POD, both in leaves and roots. Whether direct linkage exists between POD and APX in response to Hg stress in upland cotton should be further studied.
4. Materials and Methods
4.1. Assay on Seed Germination
Matured uniform-sized seeds of upland cotton standard species Texas-Marker-1 (TM-1) were decoated and sterilized by 70% ethyl alcohol for 5 min, and then treated by 0.1% (w/v) HgCl2 for 5 min. They were thoroughly washed with distilled water more than 4 times. Aiming at examining Hg tolerance extent in upland cotton, a wider range of concentration gradient was employed in germination experiments via 0, 1, 10, 100, 1000 and 10,000 µM and in the form of HgCl2. Every 20 seeds were allocated into flasks with different Hg salt concentration. The flasks were placed in dark conditions in an orbital shaker (Thermo Fisher Scientific, Waltham, MA, USA) at 120 rpm at 30 °C. The percentages of seeds germination were counted every 24 h, 3 times in total.
The germination rate was calculated as the formula:
| (1) |
4.2. Investigation on Growth and Hg Distribution
The germinating seeds were kept in a dark room at 28 ± 2 °C, 65% relative humidity, for 3 days, and then the baby seedlings were transferred to growth chambers with hydroponic solution condition (1/8 Murashige and Skoog concentration), under light/dark: 16 h/8 h, before emergence of true leaf. The seedlings were permitted to grow for 2 weeks in the hydroponic media and the solution were refreshed every 2 days. The 15-day-old seedlings were treated by HgCl2 for a week. In this experiment, the concentrations treated were chosen as 0, 1, 10, 50, 100 µM according to the germination performance responding to the previous wider dose border. Fresh and dry roots, stems and leaves were weighed from 3 individuals as a replicate, a total of 9 seedlings were used for each concentration per treatment. The inhibitory extent in seedling growth evaluated by inhibitory rate (IR, referring the following formula (2). To determine Hg content, approximate 0.2 g dry samples were proceeded by nitric acid digestion for upcoming Hg element quantification by Atomic Fluorescence Spectrometer referring to [40]. Hg concentrated in tissues and translocation calculated by formulas (3) and (4).
| (2) |
| (3) |
| (4) |
4.3. Measures of Pigments and Gas Exchanges in Leaves
Measurement of chlorophyll pigments was conducted according to the protocol of Porra [41]. To determine chlorophyll a and chlorophyll b and total chlorophyll contents, reaction solution was made using pure acetone, pure ethanol and distilled water were mixed in the ratio of 4.5: 4.5:1. The 0.5 g small leaf pieces were taken in a 25 mL tube and placed in dark until the color of the sample convert into white. Values of OD663 and OD645 were read by UV-spectral. Chlorophyll contents were determined using the following formulae:
| Chl. (a) (mg/L) = 9.78OD663 − 0.99OD645 | (5) |
| Chl. (b) (mg/L) = 21.43OD645 − 4.65OD663 | (6) |
| Chl. (t) = Chl. (a) + Chl. (b) | (7) |
where Chl. (a), Chl. (b) and Chl. (t) are abbreviated from chlorophyll a, chlorophyll b and total chlorophyll respectively.
Gas exchange parameters, including CO2 assimilation rate (Pn), stomatal conductance (Gs) and the intercellular CO2 concentration (Ci), were determined by an infrared gas analyzers portable photosynthesis system (LI-COR 6400, Lincoln, NE, USA), using third fully expanded leaves. The measurement was conducted during 10:00–11:00 a.m., maintaining the air temperature, air relative humidity, photosynthetic photon flax density (PPFD), and CO2 concentration at 25 °C, 75–85%, 1000 µMol m−2 s−1 and 400 µMol m−2 s−1, respectively.
4.4. Assay on Malondialdehyde (MDA) Content and Electrolyte Leakage (EL)
Melondialdehyde (MDA) contents were determined according to the protocol describe by Zhou [42]. Briefly, 0.5 g root or leaf samples was homogenized in tube with 10 mL 0.5% TBA, followed by heating the homogenate at 95 °C for 30 min and then cooled on ice immediately. After that, it was centrifuged at 5000 rpm for 8 min and the supernatant was taken for measure at 532 nm and 600 nm. The determination of MDA was followed as the formula:
| (8) |
where A = Volume of total reaction solution and enzyme extract, V = Total volume of PBS used, a = Volume of the enzyme extract used, W = Fresh weight of the sample and E = Constant for MDA (1.55 × 10−1).
Leaf and root samples were divided into small pieces of about 5 mm in size respectively. Electrolyte leakage (EL) was estimated referring to Dionisio-Sese and Tobita (1998) and the relative conductivity (RC) was calculated via the following formula,
| (9) |
4.5. Determination of Antioxidant Enzyme Activities in Cotton Seedlings
For the determination of antioxidant enzymes’ activities, 0.5 g fresh samples of roots or leaves were homogenized with 3 mL potassium phosphate buffer (PBS, 50 mM and pH 7.8) in prechilled mortar and pestle, and then finally the volume of the homogenate was made to be 8 mL with further addition of BPS. The homogenate was centrifuged for 20 min at 12,000 rpm at 4 °C, and the supernatant was preserved for determination of antioxidant enzymes’ activities. The assays for the determination of various antioxidants were performed according to the established protocols described by Daud [11].
4.6. Statistical Analyses Plata
In this study, the data were statistically analyzed based on one-way analysis of variance (ANOVA) by software SAS (Version 9) (SAS Institute INC, Raleigh, NC, USA). All test samples contained 3 replicates. Means were separated at 5% level of significance by least significant difference (LSD) test.
5. Conclusions
In conclusion, cotton seeds versus plants had a higher tolerance to Hg stresses. In view of the data on means, involving GR, growth parameters, pigments, gas exchanges, RC and the actives of antioxidant enzymes, significant alterations appeared within cotton seeds and seedlings, at 1000 and 10 µM Hg, respectively. In plants, relatively severe phytotoxicity and damage in response to Hg started above the 50 µM level. Hg distribution in cotton plants affected by Hg levels and root detained more Hg ion compared to shoots. As a whole, in cotton plants, the ability to concentrate and transport Hg ion seemed to close the limitation at over 50 µM concentrations. Overall, cotton seedlings showed some natural tolerance to Hg toxicity, whereas there was no apparent advantage to other field crops.
Acknowledgments
We gratefully acknowledge supports from the Analysis Center of Agrobiology and Environmental Sciences and the Institute of Agrobiology and Environmental Sciences, Zhejiang University, for in-experimental equipment.
Author Contributions
Conceptualization, S.J.; methodology, Y.Z., X.Z. (Xiujuan Zhou) and Z.Z.; software, L.M.; validation, Z.Z. and H.L.; formal analysis, Y.Z. and X.Z. (Xianwen Zhang).; investigation, L.M. and Y.Z.; resources, L.M. and S.Z.; data curation, Y.Z., H.L., Y.L.; writing—original draft prepa-ration, L.M., Y.Z., M.K.D. and J.C.; writing—review and editing, L.M. and S.Z.; visualization, L.M., X.Z. (Xianwen Zhang), X.Z. (Xiujuan Zhou), Z.Z.; supervision, S.J.; project administration, L.M. and S.Z.; funding acquisition, L.M. and S.J. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financially supported by the China Agriculture Research System, grant number CARS-18–25 and the China Postdoctoral Science Foundation, Grant number 2021M690633.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cao F., Wang R., Cheng W., Zeng F., Ahmed I.M., Hu X., Zhang G., Wu F. Genotypic and environmental variation in cadmium, chromium, lead and copper in rice and approaches for reducing the accumulation. Sci. Total. Environ. 2014;496:275–281. doi: 10.1016/j.scitotenv.2014.07.064. [DOI] [PubMed] [Google Scholar]
- 2.Suszcynsky E.M., Shann J.R. Phytotoxicity and accumulation of mercury in tobacco subjected to different exposure routes. Environ. Toxicol. Chem. 1995;14:61–67. doi: 10.1002/etc.5620140108. [DOI] [Google Scholar]
- 3.Han F.X., Banin A., Su Y., Monts D.L., Plodinec J.M. Industrial age anthropogenic inputs of heavy metals into the pedosphere. Sci. Nat. 2002;89:497–504. doi: 10.1007/s00114-002-0373-4. [DOI] [PubMed] [Google Scholar]
- 4.Heaton AC P., Rugh C.L., Wang N., Meagher R.B. Physiological responses of transgenic merA-Tobacco (Nicotiana tabacum) to foliar and root mercury exposure. Water Air Soil Pollut. 2005;161:137–155. doi: 10.1007/s11270-005-7111-4. [DOI] [Google Scholar]
- 5.Han F.X., Su Y., Monts D.L., Waggoner C.A., Plodinec M.J. Binding, distribution, and plant uptake of mercury in a soil from Oak Ridge, Tennessee, USA. Sci. Total Environ. 2006;368:753–768. doi: 10.1016/j.scitotenv.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Maggio A., Joly R.J.J. Effects of Mercuric Chloride on the Hydraulic conductivity of tomato root system. Plant Physiol. 1995;109:331–335. doi: 10.1104/pp.109.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W., Tyerman S.D. Inhibition of water channels by HgCl2 in intact wheat root cells. Plant Physiol. 1999;120:849–858. doi: 10.1104/pp.120.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenti K., Fodor F., Böddi B. Mercury Inhibits the Activity of the NADPH:Protochlorophyllide Oxidoreductase (POR) Photosynthetica. 2002;40:145–151. doi: 10.1023/A:1020143602973. [DOI] [Google Scholar]
- 9.Sahu G.K., Upadhyay S., Sahoo B.B. Mercury induced phytotoxicity and oxidative stress in wheat (Triticum aestivum L.) plants. Physiol. Mol. Biol. Plants. 2012;18:21–31. doi: 10.1007/s12298-011-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Y., Lu Y., Zhu S. Advance in studies of the mechanism of salt tolerance and controlling of salt damage in upland cotton. Cotton Sci. 2006;18:248–254. [Google Scholar]
- 11.Daud Ali S., Variath M.T., Zhu S.J. Differential physiological, ultramorphological and metabolic responses of cotton cultivars under cadmium stress. Chemosphere. 2013;3:2593–2602. doi: 10.1016/j.chemosphere.2013.09.082. [DOI] [PubMed] [Google Scholar]
- 12.Daud M.K., Quiling H., Lei M., Ali B., Zhu S.J. Ultrastructural, metabolic and proteomic changes in leaves of upland cotton in response to cadmium stress. Chemosphere. 2015;120:309–320. doi: 10.1016/j.chemosphere.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 13.Daud M.K., Mei L., Azizullah A., Dawood M., Ali I., Mahmood Q., Ullah W., Jamil M., Zhu S.J. Leaf-based physiological, metabolic, and ultrastructural changes in cultivated cotton cultivars under cadmium stress mediated by glutathione. Environ. Sci. Pollut. R. 2016;23:15551–155564. doi: 10.1007/s11356-016-6739-5. [DOI] [PubMed] [Google Scholar]
- 14.Khan M.D., Mei L., Ali B., Chen Y., Cheng X., Zhu S.J. Cadmium-Induced upregulation of lipid peroxidation and reactive oxygen species caused physiological, biochemical, and ultrastructural changes in upland cotton seedlings. Biomed. Res. Int. 2013;2013:1–10. doi: 10.1155/2013/374063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mei L., Daud M.K., Ullah N., Ali S., Khan M., Malik Z., Zhu S.J. Pretreatment with salicylic acid and ascorbic acid significantly mitigate oxidative stress induced by copper in cotton genotypes. Environ. Sci. Pollut. R. 2015;22:9922–9931. doi: 10.1007/s11356-015-4075-9. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhuri K., Choudhuri M.A. Effects of short-term NaCl salinity stress on free radical mediated membrane damage in two jute species. Indian J. Exp. Biol. 1993;3:327–331. [Google Scholar]
- 17.Liu D., Wang X., Chen Z., Xu H., Wang Y. Influence of mercury on chlorophyll content in winter wheat and mercury bioaccumulation. Plant Soil Environ. 2010;56:139–143. doi: 10.17221/210/2009-PSE. [DOI] [Google Scholar]
- 18.Chen C., Zhou Q., Bao Y., Li Y., Wang P. Ecotoxicological effects of polycyclic musks and cadmium on seed germination and seedling growth of wheat (Triticum aestivum) J. Environ. Sci. 2010;22:1966–1973. doi: 10.1016/S1001-0742(09)60347-8. [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez-Alonso J., Sierra M.J., Lominchar M.A., Millán R. Effects of mercury on the germination and growth of Quercus ilex L. seedlings. Environ. Sci. Pollut. Res. 2019;26:30930–30940. doi: 10.1007/s11356-019-06186-8. [DOI] [PubMed] [Google Scholar]
- 20.Ling T., Fangke Y., Jun R. Effect of Mercury to Seed Germination, Coleoptile Growth and Root Elongation of Four Vegetables. Res. J. Phytochem. 2010;4:225–233. doi: 10.3923/rjphyto.2010.225.233. [DOI] [Google Scholar]
- 21.Popa K., Murariu M., Molnar R., Schlosser G., Cecal A., Drochioiu G. Effect of radioactive and non-radioactive mercury on wheat germination and the anti-toxic role of glutathione. Isot. Environ. Health Stud. 2007;43:105–116. doi: 10.1080/10256010701362112. [DOI] [PubMed] [Google Scholar]
- 22.Ariani A., Barozzi F., Sebastiani L., Di Toppi L.S., Di Sansebastiano G.P., Andreucci A. AQUA1 is a mercury sensitive poplar aquaporin regulated at transcriptional and post-translational levels by Zn stress. Plant Physiol. Biochem. 2019;135:588–600. doi: 10.1016/j.plaphy.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 23.Magistrali P.R., Borges E.E.D.E., de Oliveira J.A., Faria J.M.R., Ataide G.D., do Nascimento J.F. Mercury affects aquaporins activity and germination of the embryonic axis of Schizolobium parahyba (vell.) Blake (fabaceae) Rev. Arvore. 2019;43:430601. doi: 10.1590/1806-90882019000600001. [DOI] [Google Scholar]
- 24.Oncel I., Kele Y., Ustün A.S. Interactive effects of temperature and heavy metal stress on the growth and some biochemical compounds in wheat seedlings. Environ. Pollut. 2000;107:315–320. doi: 10.1016/S0269-7491(99)00177-3. [DOI] [PubMed] [Google Scholar]
- 25.Ketavarapu S., Yathavakilla V., Caruso J.A. A study of Se-Hg antagonism in Glycine Max (soybean) roots by size exclusion and reversed phase HPLC-ICOMS. Anal. Bioanal. Chem. 2007;389:715–723. doi: 10.1007/s00216-007-1458-x. [DOI] [PubMed] [Google Scholar]
- 26.Shanker K., Mishra S., Srivastava S., Srivastava R., Daas S., Prakash S., Srivastava M.M. Effect of selenite and selenate on plant uptake and translocation of mercury by tomato (Lycopersicum esculentum) Plant Soil. 1996;183:233–238. doi: 10.1007/BF00011438. [DOI] [Google Scholar]
- 27.Ci D., Jiang D., Wollenweber B., Dai T., Jing Q., Cao W. Cadmium stress in wheat seedlings: Growth, cadmium accumulation and photosynthesis. Acta Physiol. Plant. 2009;32:365–373. doi: 10.1007/s11738-009-0414-0. [DOI] [Google Scholar]
- 28.Rodríguez E., Peralta-Videa J.R., Israr M., Sahi S.V., Pelayo H., Sánchez-Salcido B., Gardea-Torresdey J.L. Effect of mercury and gold on growth, nutrient uptake, and anatomical changes in Chilopsis linearis. Environ. Exp. Bot. 2009;65:253–262. doi: 10.1016/j.envexpbot.2008.09.014. [DOI] [Google Scholar]
- 29.Chen Z., Pan Y., Wang S., Ding Y., Yang W., Zhu C. Overexpression of a protein disulfide isomerase-like protein from Methanothermobacter thermoautotrophicum enhances mercury tolerance in transgenic rice. Plant Sci. 2012;197:10–20. doi: 10.1016/j.plantsci.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Ones M.Q., Ruiz-Díez B., Fajardo S., López-Berdonces M.A., Higueras P.L. Lupinus albus plants acquire mercury tolerance when inoculated with an Hg-resistant Bradyrhizobium strain. Plant Physiol. Bioch. 2013;73:168–175. doi: 10.1016/j.plaphy.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Zhong M., Wang Y., Hou K., Shu S., Sun J., Guo S. TGase positively regulates photosynthesis via activation of Calvin cycle enzymes in tomato. Hortic. Res. 2019;6:1–11. doi: 10.1038/s41438-019-0173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortega-Villasante C., Rellan Alvarez R.N., Francisca FDel C., Carpena-Ruize RN O., Herna Ndez L.E. Cellular damage induced by cadmium and mercury in Medicago sativa. J. Exp. Bot. 2005;56:2239–2251. doi: 10.1093/jxb/eri223. [DOI] [PubMed] [Google Scholar]
- 33.Mishra A., Choudhuri M.A. Effects of aslicylic acid on heavy metal-induced membrane deterioration mediated by lipoxygenase in rice. Biol. Plant. 1999;42:409–415. doi: 10.1023/A:1002469303670. [DOI] [Google Scholar]
- 34.Zhou Z.S., Huang S.Q., Guo K., Mehta S.K., Zhang P.C., Yang Z.M. Metabolic adaptations to mercury-induced oxidative stress in roots of Medicago sativa L. J. Inorg. Biochem. 2007;101:1–9. doi: 10.1016/j.jinorgbio.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Bhaduri A.M., Fulekar M.H. Antioxidant enzyme responses of plants to heavy metal stress. Rev. Environ. Sci. Bio/Technol. 2012;11:55–69. doi: 10.1007/s11157-011-9251-x. [DOI] [Google Scholar]
- 36.Mohammad A.H., Pukclai P., Jaime AT S., Masayuki F. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxifification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012;872875:1–37. [Google Scholar]
- 37.Israr M., Sahi S.V. Antioxidative responses to mercury in the cell cultures of Sesbania drummondii. Plant Physiol. Bioch. 2006;44:590–595. doi: 10.1016/j.plaphy.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Pedron F., Petruzzelli G., Barbafieri M., Tassi E., Ambrosini P., Patata L. Mercury Mobilization in a Contaminated Industrial Soil for Phytoremediation. Commun. Soil Sci. Plant Anal. 2011;42:2767–2777. doi: 10.1080/00103624.2011.622823. [DOI] [Google Scholar]
- 39.Moreno-Jiménez E., Alosa J.P., Esteban E., Carpena-Ruiz R.O. Mercury accumulation and resistance to mercury stress in Rumex induratus and Marrubium vulgare grown in perlite. J. Plant Nutr. Soil. Sci. 2007;170:485–494. doi: 10.1002/jpln.200625238. [DOI] [Google Scholar]
- 40.Lin H., Santa-Rios A., Barst B.D., Basu N., Bayen S. Occurrence and bioaccessibility of mercury in commercial rice samples in Montreal (Canada) Food Chem. Toxicol. 2019;126:72–78. doi: 10.1016/j.fct.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Porra R.J., Thompson W.A., Kriedemann P.E. Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls a and b Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy. Biochim. Biophys. Acta. 1989;975:384–394. doi: 10.1016/S0005-2728(89)80347-0. [DOI] [Google Scholar]
- 42.Zhou W., Leul M. Uniconazole-induced alleviation of freezing injury in relation to changes in hormonal balance, enzyme activities and lipid peroxidation in winter rape. Plant Growth Regul. 1998;26:41–47. doi: 10.1023/A:1006004921265. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.