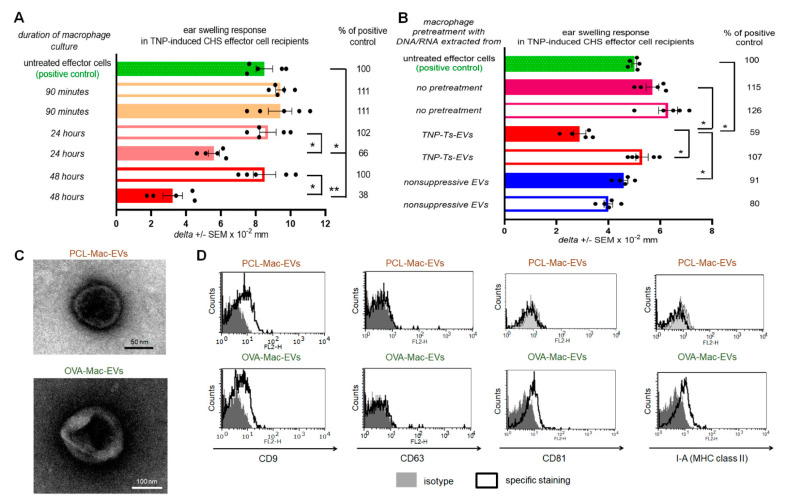
Figure 1.
Macrophages treated with Ts-EVs release inhibitory Mac-EVs that differ in marker’s expression pattern depending on mouse immunizing antigen. (A) Macrophages, after 30 min treatment at 37 °C with TNP-Ts-EVs, were cultured in protein-free MDM medium. Yielded supernatant was collected 90 min, 24 h or 48 h later, filtered and ultracentrifuged, and resulting fractions (i.e., pellet–filled bars; and supernatant above–open bars) were used to treat CHS effector cells prior to their transfer into naive recipients (n = 5 per group) that were immediately challenged with hapten to elicit CHS reaction, measured as ear swelling 24 h later. (B) Untreated macrophages or macrophages treated for 30 min at 37 °C with DNA/RNA extracted from either TNP-Ts-EVs or control, non-suppressive EVs, were cultured in protein-free MDM medium for 48 h. Yielded supernatant was filtered and ultracentrifuged, and resulting fractions (i.e., pellet–filled bars; and supernatant above–open bars) were used to treat CHS effector cells prior to their transfer into naive recipients (n = 5 per group) that were immediately challenged with hapten to elicit CHS reaction, measured as ear swelling 24 h later. (C) PCL-Mac-EVs (produced by TNP-Ts-EV-treated macrophages from PCL-sensitized mice) and OVA-Mac-EVs (produced by OVA-Ts-EV-treated macrophages from OVA-immunized mice) were absorbed onto cupper grid, negatively stained with 3% uranyl acetate, and visualized with TEM microscope. (D) PCL-Mac-EVs and OVA-Mac-EVs were coated onto latex beads, stained with fluoresceinated antibodies against selected EVs’ markers, including CD9, CD63, CD81 tetraspanins and I-A molecules, and analyzed with flow cytometry. Data are expressed as delta ± SEM. One-way ANOVA with post hoc RIR Tukey test; * p < 0.05, ** p < 0.01.