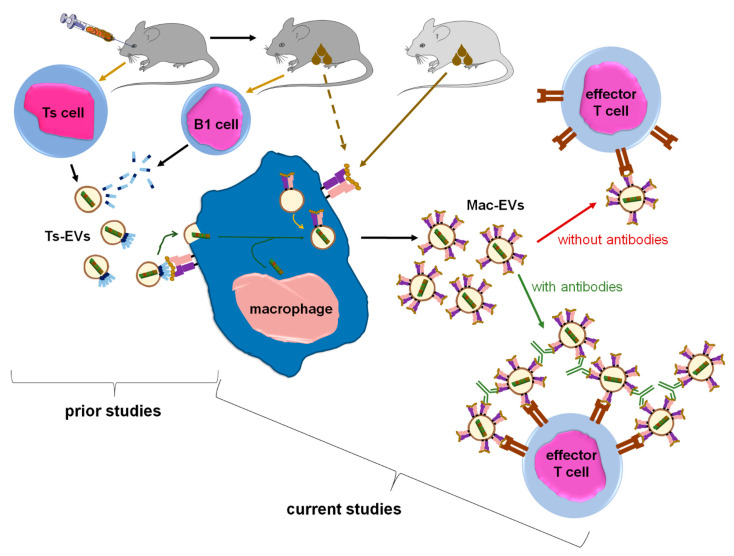
Figure 7.
Summary of the findings in the context of existing knowledge. From prior studies we knew that intravenous tolerization of mice with antigen-coupled syngeneic red blood cells induces Ts cells to release miRNA-150 in Ts-EVs that are then coated with antigen-specific antibody light chains. The latter are secreted by B1 cells activated by antigen applied to mice via cutaneous route. Subsequently, Ts-EVs target macrophages that are able to present antigenic determinant complexed with MHC class II after cutaneous immunization of donor animal. In turn, Ts-EV-targeted macrophages suppress CHS and DTH responses in mice. Currently, we have shown that macrophages treated with Ts-EV-transmitted miRNA-150 begin to release Mac-EVs that also contain miRNA-150, which finally inhibits CHS and DTH effector T cell activity. Crucially, Mac-EVs express MHC class II molecules that are likely complexing the antigenic determinant, which enables the specific targeting of effector T cells, and also binding of specific IgG antibodies by Mac-EVs. The latter finding allowed us to discover that antigen-specific antibodies aggregate Mac-EVs, which greatly enhances their suppressive activity against effector T cells. Thus, one can conclude that macrophages multiply the number of miRNA-150 copies and release them in MHC class II-positive EVs to amplify the suppressive effect and direct it against specific effector T cells, and that this effect is significantly enhanced by incubating Mac-EVs with antigen-specific antibodies.