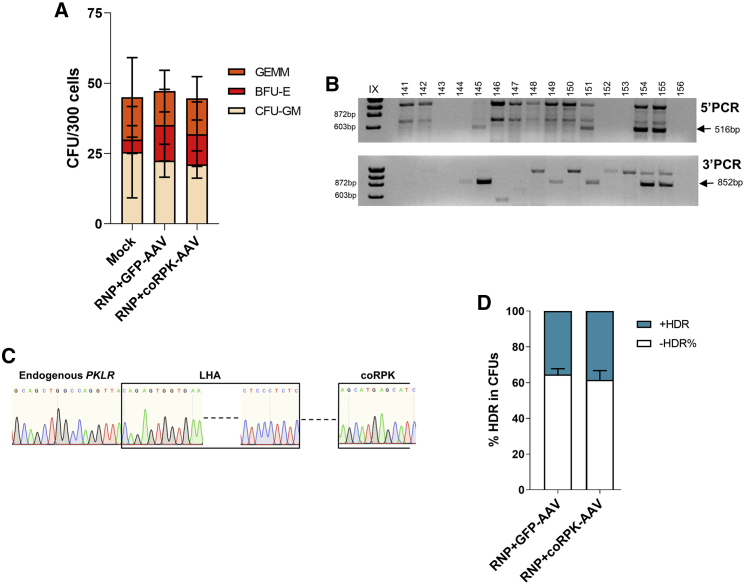
Figure 2.
The combination of SG1/Cas9 RNPs with rAAV donor delivery does not show toxicity and allows efficient gene editing in human hematopoietic progenitors at the RPK translation starting site
(A) CFU assay shows number and types of hematopoietic progenitors after gene editing. GEMM, granulocyte/erythrocyte/monocyte/megakaryocyte progenitors; BFU-E, burst forming unit-erythroid; CFU-GM, colony forming unit-erythroid. Mean ± SD is indicated. (B) Representative agarose gel of PCR analyses of the specific integration of coRPK in individually picked colonies by amplification of the left (5′ PCR, 516 bp) and right (3′ PCR, 852 bp) homology arms of the donor construct. (C) Representative Sanger sequencing of one of the positive amplicons showing correct HDR. (D) Percentage of correct HDR in CFUs assessed by PCR when cells were edited with GFP-AAV or coRPK-AAV. The figure shows the data from 3 independent experiments, in which up to 96 CFUs were analyzed per condition in each experiment. Three independent experiments were performed with pools of 3 or 4 different healthy donors per experiment. Significance was analyzed by non-parametric two-tailed Mann-Whitney or two-way ANOVA tests.