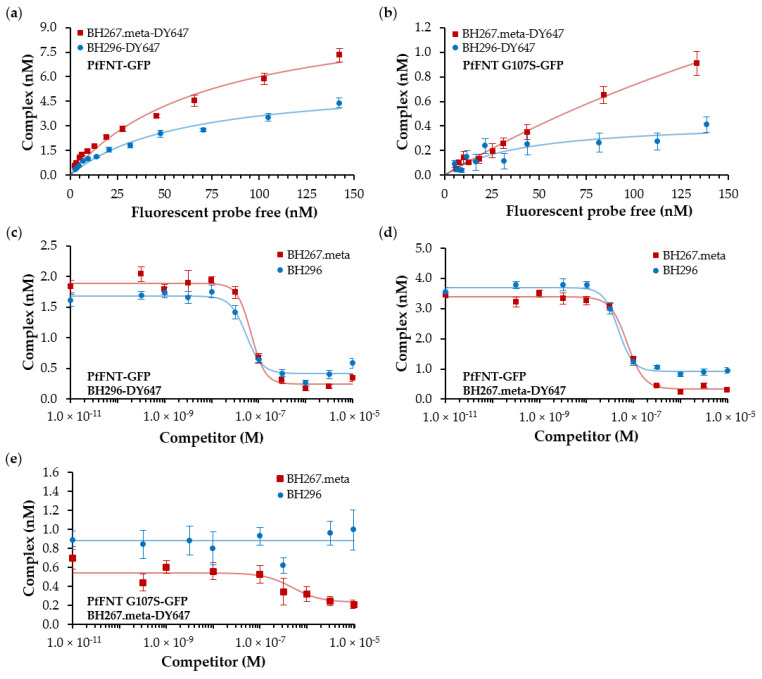
Figure 4.
Affinity determination of BH296, BH267.meta to PfFNT and PfFNT G107S, and equilibrium saturation binding of BH296 and BH267.meta to PfFNT and PfFNT G107S. (a) BH296-DY647 and BH267.meta-DY647 bound with similar affinity to PfFNT (67 nM and 72 nM, respectively), but binding to BH296-DY647 was saturated with only 50% of the target involved in interactions. BH267.meta bound to 100% of the GFP-fusions of wildtype PfFNT. (b) Saturation binding assays of BH296-DY647 to mutant PfFNT-GFP indicated no affinity above background caused by fluorescence cross talk, but BH267.meta-DY647 bound with a KD of 405 nM. (c) Membrane preparations containing 10 nM of PfFNT-GFP were incubated with 70 nM BH296-DY647, and associated complexes competed with free compounds BH267.meta and BH296 to assess their Ki values. In the absence of a competitor, 1.5 nM of complex was formed, and both compounds displaced the tracer completely with a Ki of 48 and 66 nM, respectively. (d) Using BH267.meta-DY647 as a tracer, both compounds displaced the total of 3 nM complexes with Ki values of 47 and 56 nM. (e) Membrane preparations containing GFP fusions of mutant PfFNT G107S were mixed with 150 nM of the labeled BH267.meta tracer and competed with BH267.meta and BH296. Only BH267.meta displaced the tracer with a Ki of 417 nM, whereas BH296, even at concentrations of 10 µM, did not compete in the interaction.