Table 1.
Asymmetric Synthesis of Cyclobutanones by Co(II)-Catalyzed 1,4-C–H Alkylation of α-Diazoketonesa,b,c,d
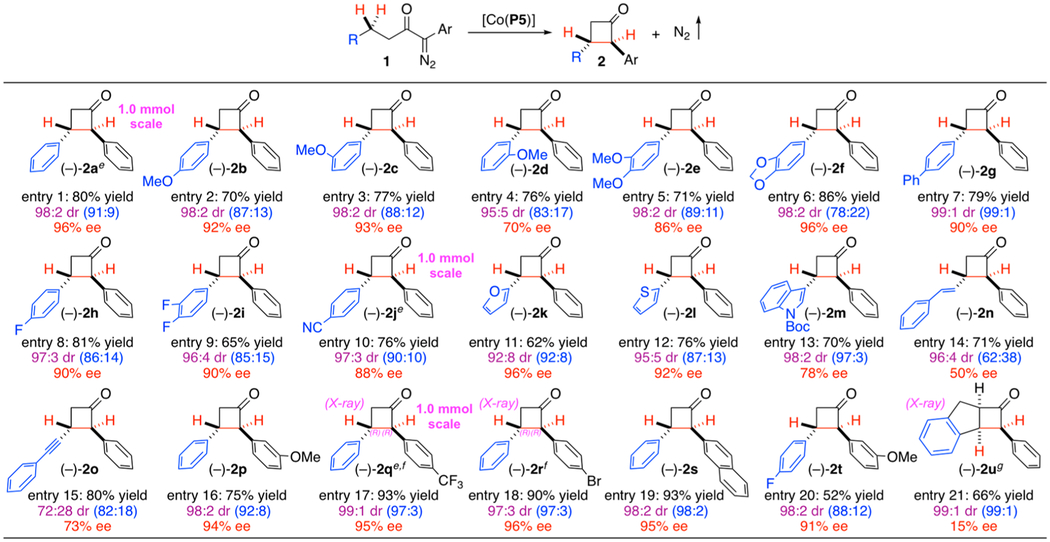
|
Carried out with 1 (0.10 mmol) and [Co(P5)] (2 mol %) in TBME (0.5 mL) at 40 °C for 12 h.
Isolated yield.
Diastereomeric ratio (dr) determined by 1H NMR analysis: trans-enriched products after purification by silica gel column chromatography due to isomerization; value in parathesis determined from reaction mixture before purification.
Enantiomeric excess (ee) of trans-diastereomer determined by chiral HPLC after purification.
Reaction performed in 1.0 mmol scale.
Absolute configuration determined by X-ray crystallography.
Relative configuration determined by X-ray crystallography.
