FIG. 3.
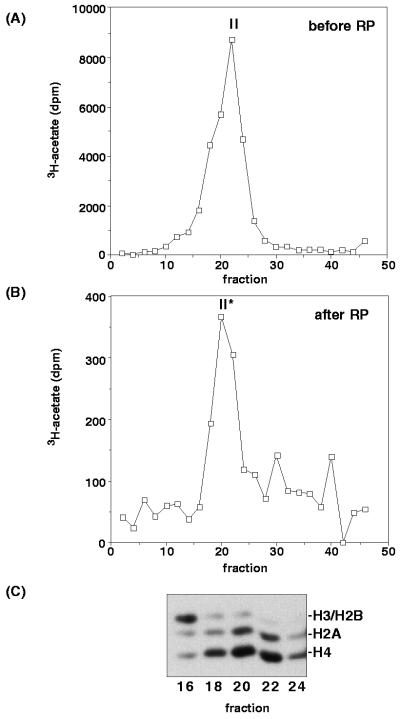
Following RP-HPLC and renaturation, the size of the H2A/H4 nucleosomal HAT activity and its substrate specificity are unchanged. (A) Sedimentation profile of HAT activity of native DNase I macronuclear extract on a sucrose-density gradient, shown for reference. (B) Sedimentation profile of HAT activity of the nucleosomal H2A/H4 HAT activity (peak II) following RP-HPLC and renaturation. In both cases, the size of the active protein/complex was estimated to be about ∼80 kDa relative to marker proteins resolved in parallel gradients (data not shown). (C) Substrate specificity of nucleosome acetylation by fractions from panel B as indicated. A fluorogram of a SDS–12.5% polyacrylamide gel is shown. Note that nucleosomal HAT activity (II*) remained H2A/H4 specific (compare to Fig. 2B).