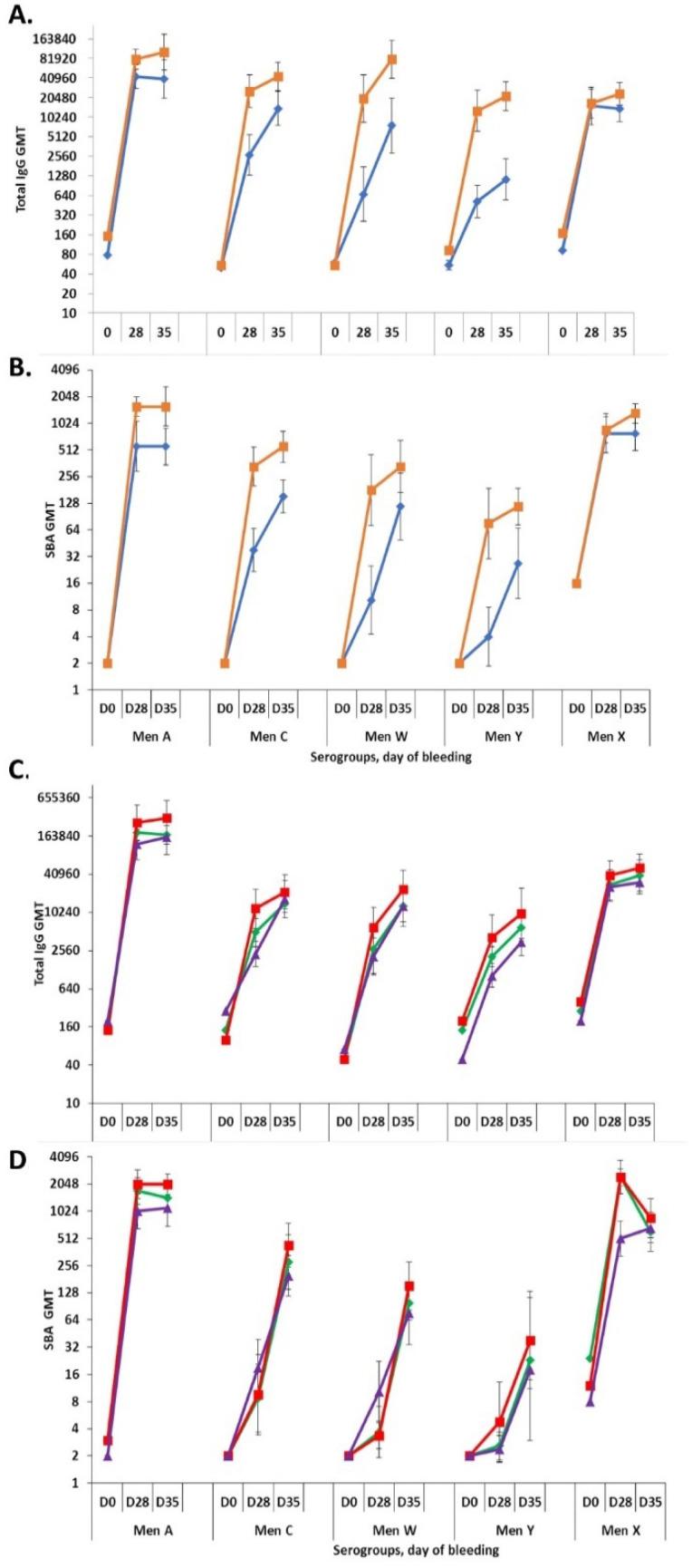
Figure 7.
Immunogenicity study in New Zealand White rabbits (eight/formulation) of NmCV-5 Phase 1 (A,B), and Phase 3 (C,D) clinical lots. Total IgG (A,C) and rabbit complement-dependent serum bactericidal activity titers (B,D) to meningococcal serogroups A, C, Y, W, and X in serum samples collected at three timepoints (Days 0, 28, and 35) were determined using the bead-based ELISA and rSBA assay. The immunogenicity of Phase 1 vaccine lots were tested as adjuvanted (■) and non-adjuvanted (♦) formulations (A,B). Phase 3 clinical consistency lots (n = 3). Lot 1 (♦), Lot2 (■), Lot3 (▲) were non-adjuvanted based upon data from the Phase 1 and 2 clinical trials [20,22] (C,D). Results are expressed as Geometric Mean Titers. Error bars represent 95% confidence intervals. Statistical significance between the adjvuanted and non-adjuvanted groups is described in the text. The study was performed at SIIPL.