Table 2.
AChE and BuChE inhibitory activity of the quinoxaline derivatives.
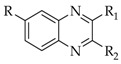
| ||||||
|---|---|---|---|---|---|---|
| Code | R | R1 | R2 | IC50 AChE (µM) |
IC50 BChE (µM)(%Inhibition) |
Selectivity BChE/AChE |
| 3a | H | H | H | 13.22 ± 4.1 | 40.64 ± 1.6 | 3.07 |
| 3b | H | Ph | H | 50.08 ± 2.6 | 14.91 ± 2.6 | 0.29 |
| 3c | H | CH3 | CH3 | 7.25 ± 1.5 | (23.42 ± 1.7%) | - |
| 4a | Cl | H | H | 23.87 ± 1.3 | (37.42 ± 2.3%) | - |
| 4b | Cl | Ph | H | 28.49 ± 1.6 | (41.16 ± 1.5%) | - |
| 4c | Cl | CH3 | CH3 | 10.67 ± 1.4 | (37.35 ± 1.3%) | - |
| 5a | NO2 | H | H | 21.31 ± 1.5 | 42.02 ± 1.0 | 1.97 |
| 5b | NO2 | Ph | H | 39.0 ± 0.8 | 60.95 ± 3.4 | 1.56 |
| 5c | NO2 | CH3 | CH3 | 8.42 ± 1.8 | (32.13 ± 1.9%) | - |
| 6a | NH2 | H | H | 0.74 ± 0.5 | (32.87 ± 2.8%) | - |
| 6b | NH2 | Ph | H | 1.31 ± 0.2 | (44.14 ± 1.0%) | - |
| 6c | NH2 | CH3 | CH3 | 0.077 ± 0.01 | (29.22 ± 1.95%) | - |
| Tacrine | 0.11 ± 0.01 | 0.0066 ± 0.001 | 0.09 | |||
| Galanthamine | 0.59 ± 0.13 | 11.55 ± 5.5 | 19.58 | |||
IC50 values are expressed as the mean ± SD (n = three independent experiments). %inhibition at 100 µM are shown in square brackets as the mean ± SD (n = three independent experiments). Selectivity index for AChE: IC50 BChE/IC50 AChE.
