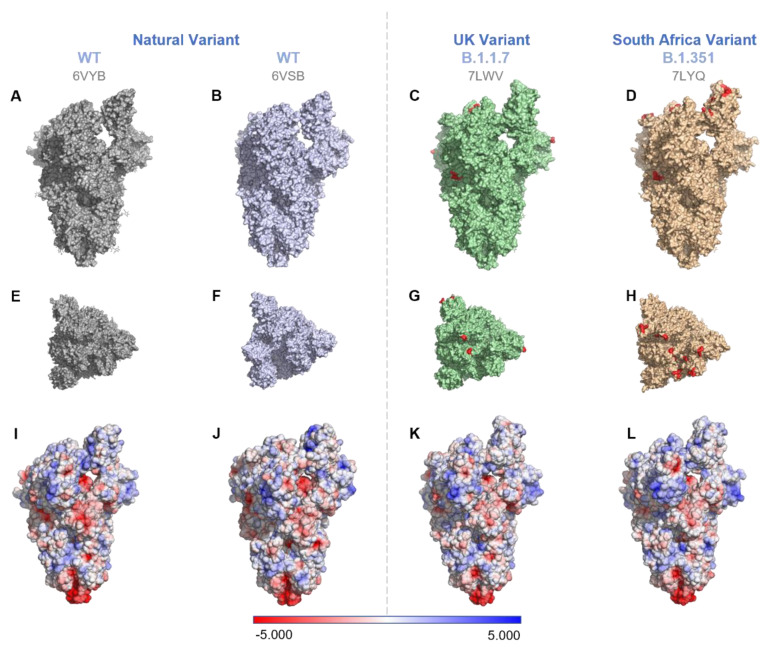
Figure 2.
Comparison of the mutations (Rows 1 and 2) and surface electrostatic potentials (Row 3) on spike proteins of two VOIs with respect to the WT. WT (PDB ID: 6VYB [24] (1st column) and 6VSB [25] (2nd column)), B.1.1.7 (PDB ID: 7LWV [22]), and B.1.351 (PDB ID: 7LYQ [22]). (A–D) show the side view, 1st row; (E–H) show the top view, 2nd row. For the VOIs, the AA mutations on the S proteins (see Table 2 for details) are marked red with one residue before and after the actual mutational site for better visibility. (I–L) S-protein surface potentials of the WT and VOIs. The surface potentials were generated by preparing molecules with the pdb2pqr method and applying APBS electrostatics using PyMol v2.4.1.