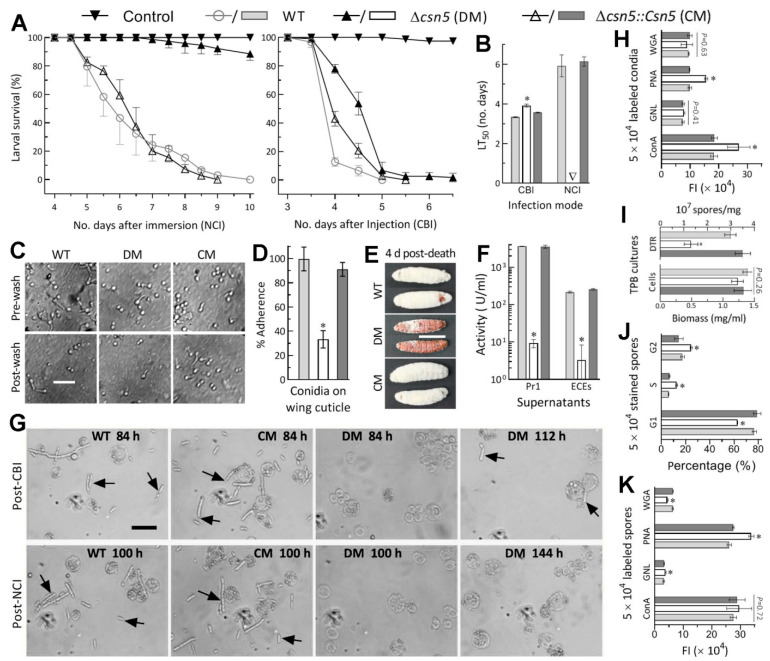
Figure 2.
Essential role of Csn5 in the infection cycle and related cellular events of B. bassiana. (A,B) Time–survival trends of G. mellonella larvae after topical application (immersion) of a 107 conidia/mL suspension for normal cuticle infection (NCI) and intrahaemocoel injection of ~500 conidia per larva for cuticle-bypassing infection (CBI) and LT50s (number of days) estimated from the trends. (C,D) Microscopic images (scale bar: 20 μm) for conidia attached to locust hind wings pre-wash and post-wash and conidial adherence assessed as percent ratios of pre-wash counts over post-wash counts with respect to the WT standard, respectively. (E) Images (scale bar: 10mm) of hyphal outgrowths on the surfaces of insect cadavers 4 days post-death via CBI. (F) Total activities of cuticle-degrading ECEs and Pr1 proteases quantified from the supernatants of 3-day-old CDB-BSA cultures, which were initiated by shaking 106 conidia/mL suspensions at 25 °C. (G) Microscopic images (scale bar: 20 μm) for the presence and abundance of hyphal bodies (arrowed) and host haemocytes (spherical or subspherical cells) in haemolymph samples taken from surviving larvae post-NCI or post-CBI. Note that the ∆csn5 mutant failed to produce hyphal bodies 144 h post-NCI and produced very few 110 h post-CBI. (H) Carbohydrate epitope patterns of conidia labelled with four fluorescence lectins. (I) Biomass levels and dimorphic transition rates measured from 3-day-old TBP cultures mimicking insect haemolymphs. (J,K) Distribution of DNA profiles for the cell cycle phases of DNA-stained blastospores in the TPB cultures and carbohydrate epitope patterns of those blastospores labelled with four fluorescence lectins, respectively. * p < 0.05 (B), * p < 0.01 (H,J,K), or * p < 0.001 (D,F,I). Error bars: SDs of the means from three independent replicates.