Table 1.
Structures and antiplasmodial activity of bisindolylcyclobutenediones 2a–ar and bisindolylmaleimide 8 a.
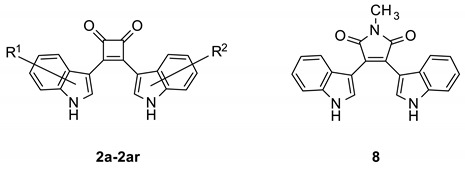
| ||||
|---|---|---|---|---|
| Entry | R1 | R2 | IC50 [µM] b | Inhibition at 3 µM [%] c |
| 2a | H | H | 8.33 | 17.9 ± 3.3 |
| 2b | H | 5-Br | 3.02 | 65.9 ± 1.8 |
| 2c | H | 5-Cl | 4.23 | 44.7 ± 0.5 |
| 2d | H | 5-OCH3 | n.d. | 18.3 ± 5.0 |
| 2e | H | 5-OCH2Ph | n.d. | −4.43 ± 10.6 |
| 2f | H | 5-CN | 6.72 | 26.8 ± 1.56 |
| 2g | H | 2-CH3 | >30 | 23.4 ± 5.4 |
| 2h | H | 2-Ph | 6.38 | 65.0 ± 2.8 |
| 2i | 1-CH3 | 1-CH3 | n.d. | 3.53 ± 1.7 |
| 2k | H | 1-[3-(dimethyl-amino)propyl] | 0.376 | 98.1 ± 1.8 |
| 2l | 2-CH3 | 2-CH3 | n.d. | 18.3 ± 6.3 |
| 2m | 2-Ph | 2-Ph | 2.47 | 41.1 ± 0.6 |
| 2n | 5-OCH3 | 5-OCH3 | n.d. | 19.7 ± 3.6 |
| 2o | 5-Br | 5-Br | >30 | n.d. |
| 2p | 5-CN | 5-CN | n.d. | −3.10 ± 0.9 |
| 2q | H | 1-CH3 | 2.97 | 52.0 ± 1.8 |
| 2r | H | 5-I | 0.915 | 31.3 ± 2.7 |
| 2s | 2-Ph | 5-Br | n.d. | 16.0 ± 0.7 |
| 2t | 5-Br | 1-CH3 | n.d. | 1.38 ± 3.3 |
| 2u | 2-Ph | 1-CH3 | 1.72 | 67.7 ± 0.7 |
| 2v | 5-OCH3 | 2-CH3 | 10.0 | 34.2 ± 8.0 |
| 2w | 2-Ph | 2-CH3 | 9.26 | 41.1 ± 1.7 |
| 2x | 5-Br | 2-CH3 | >30 | 45.6 ± 1.8 |
| 2y | 1-CH3 | 2-CH3 | 2.67 | 59.6 ± 0.2 |
| 2z | 5-Br, 1-CH3 | 2-CH3 | n.d. | 12.7 ± 2.8 |
| 2aa | 2-Ph | 5-OCH3 | n.d. | 22.4 ± 2.7 |
| 2ab | 5-F | 5-OCH3 | n.d. | 19.2 ± 3.1 |
| 2ac | 5-Cl | 5-OCH3 | 1.52 | 87.0 ± 0.5 |
| 2ad | 5-Br | 5-OCH3 | 0.47 | 98.8 ± 0.7 |
| 2ae | 5-I | 5-OCH3 | 0.64 | 99.1 ± 0.3 |
| 2af | 1-CH3 | 5-OCH3 | n.d. | 13.6 ± 3.9 d |
| 2ag | 5-Br, 1-CH3 | 5-OCH3 | n.d. | −0.06 ± 2.43 |
| 2ah | 5-CN | 5-OCH3 | 6.92 | 26.8 ± 1.1 |
| 2ai | 7-Cl | 5-OCH3 | 0.691 | 84.5 ± 1.0 |
| 2aj | 7-Br | 5-OCH3 | 0.296 | 99.5 ± 0.4 |
| 2ak | 7-I | 5-OCH3 | 0.116 | 98.8 ± 1.2 |
| 2al | 7-C2H5 | 5-OCH3 | 0.511 | 87.0 ± 0.5 |
| 2am | 6-Br | 5-OCH3 | n.d. | 9.55 ± 5.8 |
| 2an | 4-Br | 5-OCH3 | n.d. | 25.7 ± 2.7 |
| 2ao | 5-Br | 1-CH3, 5-OCH3 | 0.504 | 95.4 ± 1.1 |
| 2ap | 5-Br | 5-OH | n.d. | 2.52 ± 1.4 |
| 2aq | 5-I | 5-I | n.d. | 1.79 ± 1.8 |
| 2ar | 5-OBz | 5-OCH3 | n.d. | 21.7 ± 2.5 |
| 8 | - | - | 4.0 | 34.6 ± 0.8 |
| BSD e | - | - | 0.288 | 97.6 ± 0.5 |
a n.d. = not determined. b Concentration [µM] for 50% inhibition of P. falciparum (NF54-luc strain) erythrocytic stages. c Inhibition of P. falciparum (NF54-luc strain) erythrocytic stages at 3 µM. d determined at 30 µM. e Blasticidin (BSD) was used as the positive control.
