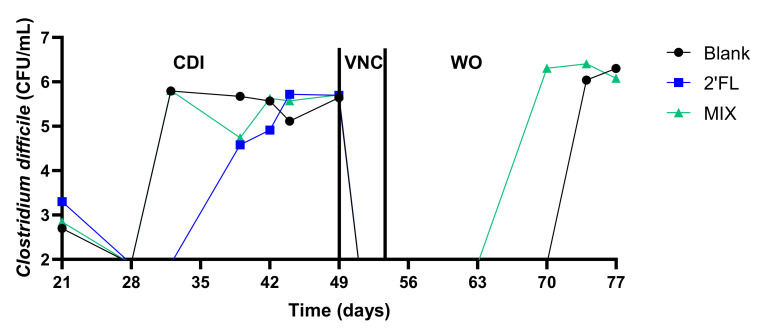
Figure 6.
C. difficile levels (log10 (CFU/mL)) during the long-term pathogutTM study (11 weeks) (test 2). Upon inoculation and stabilization of the fecal inoculum of donor A during the two weeks preceding the study (−14–0 days), a control period (0–14 days) was followed by a clindamycin treatment period (CLI: 14–21 days) which resulted in C. difficile infection (CDI: 21–49 days). From then on, while the blank was treated with vancomycin (VNC: 49–54 days), the other two arms additionally received 2′FL and MIX, respectively. 2′FL and MIX were further administered during the washout period (WO: 54–77 days). 2′FL = 2′-O-fucosyllactose, LNnT = lacto-N-neotetraose, MIX = 4:1 mixture of 2′FL/LNnT.