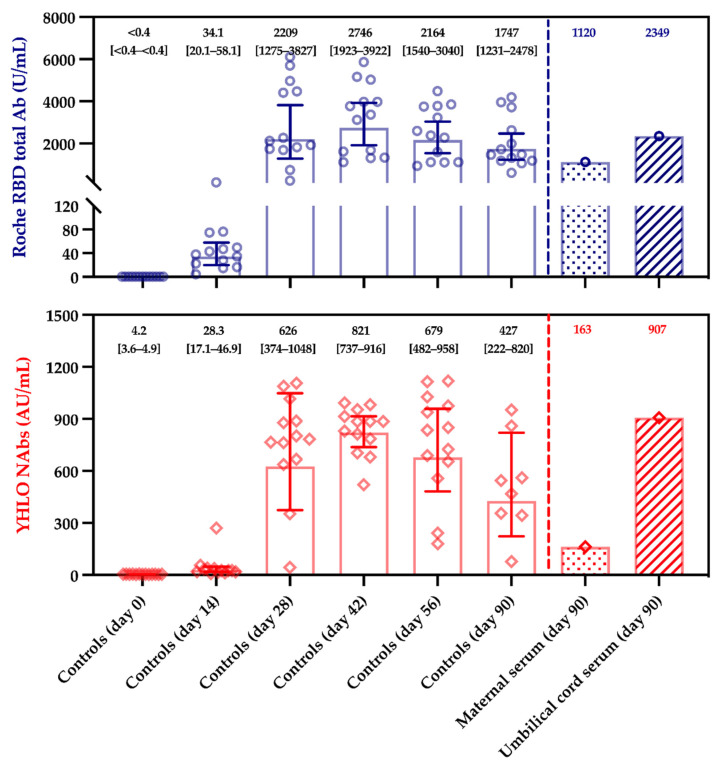
Figure 1.
Serological response in controls, maternal and umbilical cord sera. Control sera selected from the CRO-VAX HCP study cohort and included 13 non-pregnant women aged from 25 to 35 (median, 30 years of age; min–max range, 25–35), without history of SARS-CoV-2 infection. Blood was collected at baseline (day 0), day 14, day 28, day 42, day 56, and day 90. Maternal and umbilical cord blood were only collected on day 90 after the vaccination, at the time of delivery. Positivity thresholds for the Roche RBD total Ab and the YHLO NAbs assays were 0.8 U/mL and 10.0 AU/mL, respectively.