Abstract
Context
Aggressive pituitary tumors that have progressed following temozolomide have limited treatment options. Peptide receptor radionuclide therapy and immunotherapy may have a complementary role in the management of these tumors.
Methods
We provide follow-up data on a previously reported patient with a hypermutated recurrent tumor. The patient in this report provided written informed consent for tumor sequencing and review of medical records on an institutional review board–approved research protocol (NCT01775072).
Results
This patient with a corticotroph pituitary carcinoma with alkylator-induced somatic hypermutation has remained on treatment with ipilimumab and nivolumab for 3.5 years and remains clinically well. After an initial partial response to checkpoint inhibitors, she has had several recurrences that have undergone immunoediting of subclonal mutations, which have been effectively treated with continuation of immunotherapy, surgery, external beam radiation, and 177Lu-DOTATATE. Following external beam radiotherapy (RT), she had radiographic evidence of an abscopal response at a distant site of disease suggesting a synergism between checkpoint inhibitors and RT. Following treatment with 177Lu-DOTATATE, the patient had a partial response with a 61% reduction in volume of the target lesion.
Conclusion
In patients with aggressive pituitary tumors, treatment with checkpoint inhibitors may trigger an abscopal response from RT. With appropriate selection, an additional efficacious treatment, 177Lu-DOTATATE, may be available for a limited number of patients with aggressive pituitary tumors, including patients who have progressed on temozolomide and exhibit increased somatostatin receptor expression on 68Ga-DOTATATE positron emission tomography.
Keywords: checkpoint inhibitor, immunotherapy, PRRT, pituitary carcinoma
While there are retrospective data supporting the use of temozolomide in the treatment of aggressive pituitary adenomas and carcinomas, there remains a significant unmet need for effective treatments.
The largest cohort of patients receiving temozolomide in the literature is an electronic survey of the European Society of Endocrinology, which captured 166 individual patients and reported an objective response (complete or partial) in 37%—implying that a significant subset of patients failed to demonstrate a benefit [1]. Of patients who responded, the average time to progression after cessation of therapy was short, only 12 months, and these patients rarely reresponded to temozolomide when rechallenged. Of 18 patients who were rechallenged in this situation for whom there are data, only 2 of 18 had a partial response.
Recently, there has been interest in the use of checkpoint inhibitors in the management of patients with aggressive pituitary tumors, based on an earlier report describing the present patient’s dramatic response to immunotherapy [2]. There are now additional examples in the literature of patients who responded to checkpoint inhibitors [3, 4] and preclinical evidence supporting its use [5].
Peptide receptor radionuclide therapy (PRRT) with radiolabeled octreotide derivatives, such as 177Lu-DOTATATE, is an efficacious and approved therapy for gastroenteropancreatic neuroendocrine tumors [6]. 177Lu-DOTATATE delivers a cytotoxic dose of radiation to tumors with overexpression of somatostatin receptors. The requisite somatostatin density can be established with radiolabeled diagnostic tracers, such as 68Ga-DOTATATE, when tumoral uptake is equal to or higher than the normal liver. PRRT is generally well tolerated with mild and reversible toxicity. Toxicity can be short term, such as moderate fatigue, nausea (more rarely vomiting), abdominal pain, or exacerbation of hormonal syndrome such as diarrhea in carcinoid syndrome; mid term, such as hematological toxicity and transaminitis, which is mild in the large majority; and long term, such as the rare occurrence of myeloproliferative diseases and the almost exceptional occurrence of renal failure. PRRT has been tested in a few cases of treatment-refractory pituitary adenomas naive to temozolomide, with therapeutic efficacy [7, 8]. None of the reported patients who were previously treated with temozolomide and subsequently challenged with PRRT have had reported benefit [9].
In this report, we provide long-term follow-up on this previously reported patient, who remains clinically well without a new neurologic deficit and a Karnofsky Performance Score of 80, after 3.5 years on immunotherapy. Though this patient has had multiple additional recurrences, she has remained on checkpoint inhibitors, has had an abscopal response to external beam radiotherapy (RT) mediated by immunotherapy, and is the first patient with a partial response to PRRT following progression on temozolomide.
Case Description
The patient is a 45-year-old woman with an adrenocorticotropic hormone (ACTH)-secreting pituitary carcinoma described previously [2]. She initially presented with a sixth nerve palsy, facial fulness, and hirsutism. Imaging revealed an invasive pituitary macroadenoma, for which she underwent resection September 12, 2011, revealing an ACTH-secreting adenoma. Owing to rapid regrowth, she underwent reresection in 2012, followed by fractionated RT. She underwent 2 additional transsphenoidal resections in 2014 and 2015. Because of tumor growth and inadequate biochemical control on pasireotide, ketoconazole, and cabergoline, treatment with temozolomide and capecitabine (CAPTEM) was initiated in March 2016. She received 3 cycles of CAPTEM with a radiographic response; however, treatment had to be discontinued because of multiple complications including pulmonary embolus and acute renal failure. She continued to suffer from the sequelae of hypercortisolemia and was referred for adrenalectomy. As a part of preoperative testing, she received a computed tomography scan of the abdomen in May 2017, which revealed a hepatic mass. She underwent bilateral adrenalectomy and biopsy of this liver lesion June 15, 2017, which revealed a high-grade neuroendocrine neoplasm with focal positivity for ACTH. Next-generation sequencing of the temozolomide-naive pituitary adenoma and the liver metastasis revealed MSH6 inactivation and the development of alkylator-induced somatic hypermutation in the liver metastasis, as previously reported [2]. This prior report demonstrated that the mutagenesis induced by alkylator therapy led to pathway activation of the PI3K pathway through the development of a subclonal PIK3CA G1050D hot spot mutation. It was also demonstrated that the majority of the mutations in this hypermutated liver metastasis were subclonal [2, 10].
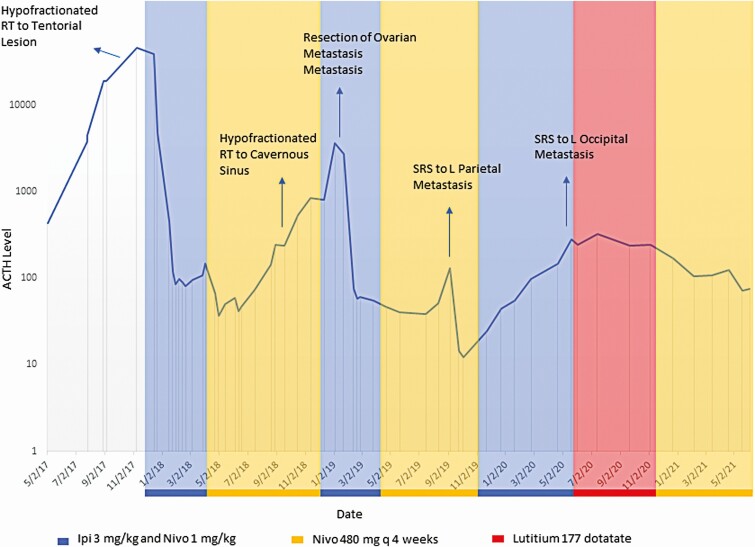
Following identification of the liver metastasis, the patient was fully restaged and found to have progressive disease within the right cavernous sinus, right Meckel cave, and right tentorium. The rapid progression of disease following bilateral adrenalectomy was consistent with Nelson syndrome. At this time, the patient was rechallenged with 2 cycles of CAPTEM; subsequent imaging demonstrated growth of the known metastasis and the development of new liver metastases, and, therefore, treatment with carboplatin and etoposide was initiated. Because of further progression after 2 cycles of carboplatin and etoposide, she received palliative RT to the tentorial component of her intracranial disease, and then was challenged with the checkpoint inhibitors, ipilimumab and nivolumab December 15, 2017. She received a 5-dose course of ipilimumab (3 mg/kg) and nivolumab (1 mg/kg), with a reduction in her plasma ACTH from a peak of 45 551 to 451 pg/mL (Fig. 1). By June 2018, her ACTH had decreased to 41.5 pg/mL and she had a dramatic reduction in disease burden both intracranially and extracranially on single-agent nivolumab.
Figure 1.
Adrenocorticotropin (ACTH) levels over the course of treatment with immunotherapy, surgery, and radiotherapy.
By July 2018, all lesions demonstrated marked improvement. At this time, her plasma ACTH increased from a nadir of 37 to 75.3 pg/mL, and there was a slight increase in the primary site of her disease (the small residual within the cavernous sinus). At this time, she was treated with a course of proton RT 25.09 cobalt Gray equivalent in 5 fractions. Unfortunately, her ACTH continued to rise to a level of 3680.7 pg/mL in the setting of a negative 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography scan that showed a subtle focus of FDG uptake at the primary site in the sella but no other concerning sites of abnormal FDG uptake (uptake in the left adnexa was believed to be physiologic). Owing to this unexplained rise in plasma ACTH, a 68Ga-DOTATATE PET/magnetic resonance (MR) scan was performed that identified abnormal uptake in the left adnexa consistent with recurrent tumor. A salpingo-oophorectomy was performed revealing a high-grade malignant neuroendocrine neoplasm, with patchy ACTH staining and following surgery, her plasma ACTH level dropped down to 76.2 pg/mL. Sequencing of the recurrent tumor was performed using the US Food and Drug Administration–authorized, Clinical Laboratory Improvement Amendments–certified next-generation sequencing platform, Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT), showing a decrease in the tumor mutational burden to 3.5 mutations/megabase (in contrast to 93 mutations/megabase in the hypermutated liver metastasis that responded to checkpoint inhibitors). She was then rechallenged with another 4-dose course of ipilimumab and nivolumab followed by single-agent nivolumab. With this rechallenge of dual checkpoint inhibitors, followed by single-agent nivolumab, her ACTH stabilized/downtrended (60.4-51.0 pg/mL; see Fig. 1), shrinkage of the disease in her skull base was observed, and no new disease was noted until August 2019.
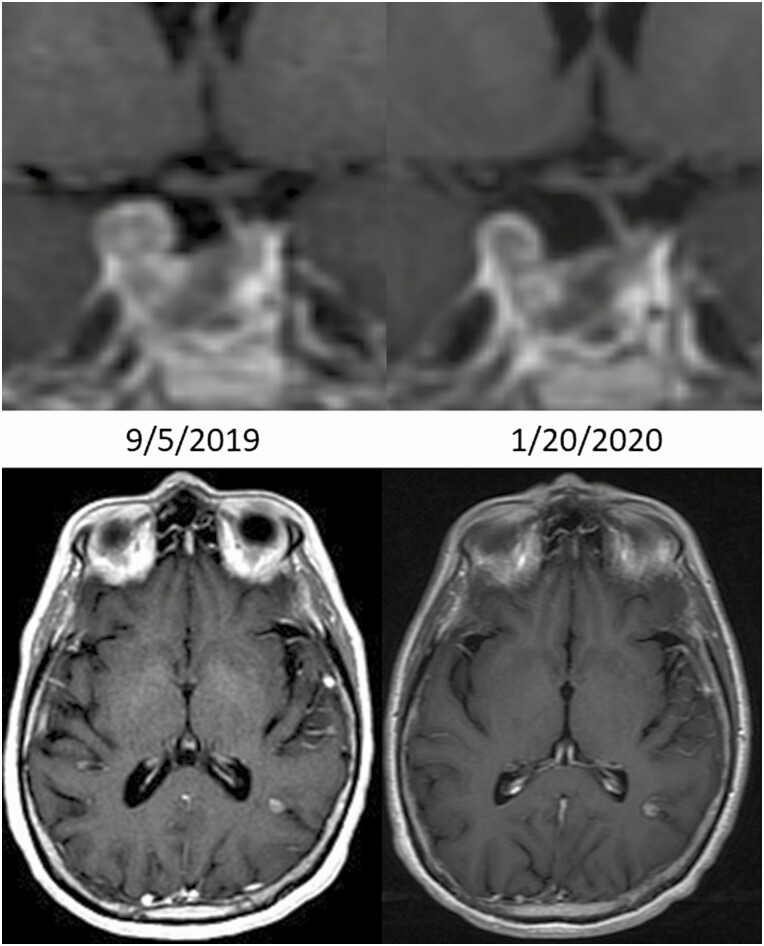
In August 2019, a new left parietal metastasis appeared and the patient’s plasma ACTH rose to 129.3 pg/mL. She received stereotactic radiosurgery to this isolated recurrence in the left parietal lobe (21 Gy in 1 fraction) September 17, 2019, that was complicated almost immediately by severe fatigue and orthostasis (seated: heart rate 112, blood pressure 104/66 and standing: heart rate 154, blood pressure undetectable). She was started on stress dose steroids with improvement in these symptoms and her ACTH unexpectedly dropped from 129.3 to 19.3 pg/mL on a physiologic dose of steroids (see Fig. 1). Notably, the component of her pituitary tumor in the cavernous sinus, which was outside the radiation field, regressed in the setting of being stable in size since the beginning of the year (Fig. 2). This is consistent with an abscopal effect as radiation induced an immunologic response that led to regression of a tumor that was distant to the site of radiation.
Figure 2.
Abscopal response in the cavernous sinus disease following radiotherapy to the left parietal metastasis September 17, 2019.
From November 5, 2019 to April 21, 2020, she received 4 additional doses of ipilimumab and nivolumab (doses 10-13). In December 2019, while on ipilimumab and nivolumab, there was transient enlargement and then shrinkage of a segment 2 liver metastasis. Unfortunately, on the magnetic resonance imaging (MRI) scan in April 2020, there was growth of the tumor in the right cavernous sinus and she was found to have a progressive left occipital lobe metastasis, while her systemic disease remained stable. She underwent stereotactic radiosurgery to this left occipital lobe metastasis in May 2020 (21 Gy in 1 fraction). Because she had already received 2 courses of RT to the skull base, she was not felt to be a candidate for further external beam RT at this site. For that reason, a repeat 68Ga-DOTATATE PET/MR was performed that revealed high avidity of the recurrent skull base tumor for the radiotracer (maximal standardized uptake value of 24.3) and low-grade uptake in the treated left parietal and left occipital brain metastases (standardized uptake value of 4.8 and 5.8, respectively). There was no focal uptake in the liver.
A decision was made to hold checkpoint inhibitors and treat the patient with 4 cycles of 177Lu-DOTATATE. She received 7.05 GBq (190.5 mCi) June 4, 2020; 6.92 GBq (187 mCi) July 27, 2020; 6.97 GBq (188.42 mCi) September 22, 2020; and 7.13 GBq (192.8 mCi) November 17, 2020, for a cumulative activity of 28.07 GBq (758.7 mCi). She tolerated treatment well without side effects; she did not experience gastrointestinal side effects, myelosuppression, transaminitis, or changes in kidney function. In fact, she felt less fatigued during treatment with 177Lu-DOTATATE, while off checkpoint inhibitors.
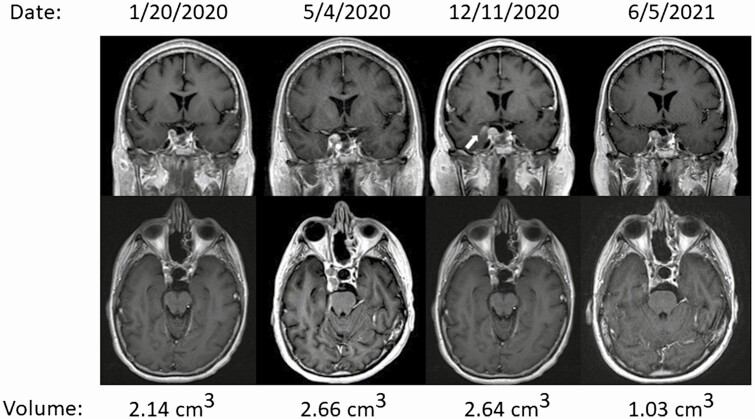
Whereas the volume of disease in her right cavernous sinus increased from 2.14 cm3 to 2.66 cm3 in the 4 months before the initiation of 177Lu-DOTATATE (as determined by a board-certified neuroradiologist through manual segmentation using iNtuition 4.4.13, TeraRecon), the volume of disease in her cavernous sinus stabilized during treatment with a final volume of 2.64 cm3 immediately following the fourth dose of 177Lu-DOTATATE. Following the fourth dose of 177Lu-DOTATATE, in the brain adjacent to the cavernous sinus, the patient did develop a small asymptomatic hemorrhage, which improved on subsequent MRI scans (Fig. 3, arrow). During treatment with 177Lu-DOTATATE from May 2020 to December 2020, a biochemical response was noted with a decrease in the plasma ACTH level from 280.5 to 171.6 pg/mL. Following the completion of 177Lu-DOTATATE, nivolumab was resumed and after 6 months further shrinkage was observed with a calculated volume of 1.03 cm3 on the MRI scan from June 5, 2021 (a volume reduction of 61% from the pre-PRRT baseline; see Fig. 3) and the plasma ACTH has declined further to 75.4 pg/mL (Fig. 1). No new sites of disease have developed.
Figure 3.
Partial response to 177Lu-DOTATATE following the initiation of treatment in June 2020 complicated by a small asymptomatic hemorrhage into the right mesiotemporal lobe (arrow).
Discussion
This case suggests that treatment response of pituitary carcinomas to checkpoint inhibitors may be associated with high tumor mutational burden, that recurrence following treatment with checkpoint inhibitor may be due to the immunoediting of subclonal mutations, and that radiation and checkpoint inhibitors can be complementary in the treatment of aggressive pituitary tumors. In this patient, an abscopal response was observed within this patient’s skull base disease following treatment of a distant brain metastasis with external beam radiation. It is less clear whether the checkpoint inhibitor mediated this patient’s treatment response to PRRT, but notably, this is the first patient to demonstrate a response to PRRT after progressing on temozolomide [7, 9]. In a recent review of the literature, among the 20 patients with aggressive pituitary tumors who received PRRT, the only patients who derived a clinical benefit from PRRT were temozolomide naive [9]. Among the 7 patients in this cohort of 20 who were temozolomide naive, 3 had a partial response and 3 had stable disease.
It is well known that the response to PRRT is mediated by overexpression of somatostatin receptors on the tumor cells, which serve as intracellular transporters for the radioactivity [11]. In this patient with a high density of somatostatin receptors at the only site of active disease as determined by 68Ga-DOTATATE PET/MR, it is possible that radiation-related cell lysis uncovered antigenic sites that then augmented the activity of immunotherapy, as has been previously demonstrated for external and internal RTs [12, 13]. It is also possible that the benefit this patient received from PRRT is directly attributable to the high dose of radiation that was administered, and that the limited activity that has been previously reported in patients with aggressive pituitary tumors who were previously treated with temozolomide is in large part due to patient selection.
Finally, this case suggests that patients who have derived a benefit from ipilimumab and nivolumab may derive continued benefit from immunotherapy (with the addition of RT and surgery) following progression in part due to abscopal effects, and possibly may benefit from a rechallenge of dual checkpoint inhibitors, as there was evidence of immune activation when this patient was retreated with combination therapy.
Acknowledgments
Financial Support: This work was supported in part by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Glossary
Abbreviations
- ACTH
adrenocorticotropic hormone
- CAPTEM
temozolomide and capecitabine
- FDG
18F-fluorodeoxyglucose
- MRI
magnetic resonance imaging
- PRRT
peptide receptor radionuclide therapy
- RT
radiotherapy
Additional Information
Disclosures: A.L.L. reports research funding to institution from Bristol Myers Squibb. V.T., M.C., J.C., T.J.Y., M.R., V.A.R., and E.B.G. have nothing to disclose. R.J.Y. has consulted for Agios, Puma, NordicNeuroLab, and ICON plc, and has research funding to his institution from Agios. L.B. has been an unpaid consultant/speaker for AAA-Novartis, Ipsen, ITM, Clovis Oncology, IBA, and has received a grant from AAA-Novartis.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. McCormack A, Dekkers OM, Petersenn S, et al. ; ESE survey collaborators. Treatment of aggressive pituitary tumours and carcinomas: results of a European Society of Endocrinology (ESE) survey 2016. Eur J Endocrinol. 2018;178(3):265-276. [DOI] [PubMed] [Google Scholar]
- 2. Lin AL, Jonsson P, Tabar V, et al. . Marked response of a hypermutated ACTH-secreting pituitary carcinoma to ipilimumab and nivolumab. J Clin Endocrinol Metab. 2018;103(10):3925-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Majd N, Waguespack SG, Janku F, et al. . Efficacy of pembrolizumab in patients with pituitary carcinoma: report of four cases from a phase II study. J Immunother Cancer. 2020;8(2):e001532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duhamel C, Ilie MD, Salle H, et al. . Immunotherapy in corticotroph and lactotroph aggressive tumors and carcinomas: two case reports and a review of the literature. J Pers Med. 2020;10(3):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kemeny HR, Elsamadicy AA, Farber SH, et al. . Targeting PD-L1 initiates effective antitumor immunity in a murine model of Cushing disease. Clin Cancer Res. 2020;26(5):1141-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hope TA, Bodei L, Chan JA, et al. . NANETS/SNMMI consensus statement on patient selection and appropriate use of 177Lu-DOTATATE peptide receptor radionuclide therapy. J Nucl Med. 2020;61(2):222-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giuffrida G, Ferraù F, Laudicella R, et al. . Peptide receptor radionuclide therapy for aggressive pituitary tumors: a monocentric experience. Endocr Connect. 2019;8(5):528-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Novruzov F, Aliyev JA, Jaunmuktane Z, Bomanji JB, Kayani I. The use of (68)Ga DOTATATE PET/CT for diagnostic assessment and monitoring of (177)Lu DOTATATE therapy in pituitary carcinoma. Clin Nucl Med. 2015;40(1):47-49. [DOI] [PubMed] [Google Scholar]
- 9. Ilie MD, Lasolle H, Raverot G. Emerging and novel treatments for pituitary tumors. J Clin Med. 2019;8(8):1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin AL, Donoghue MTA, Wardlaw SL, et al. . Approach to the treatment of a patient with an aggressive pituitary tumor. J Clin Endocrinol Metab. 2020;105(12):3807-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bodei L, Ferone D, Grana CM, et al. . Peptide receptor therapies in neuroendocrine tumors. J Endocrinol Invest. 2009;32(4):360-369. [DOI] [PubMed] [Google Scholar]
- 12. Prasad V, Zengerling F, Steinacker JP, et al. . First experiences with 177Lu-PSMA therapy in combination with pembrolizumab or after pretreatment with olaparib in single patients. J Nucl Med. 2021;62(7):975-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Azghadi S, Daly ME. Radiation and immunotherapy combinations in non-small cell lung cancer. Cancer Treat Res Commun. 2021;26:100298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.