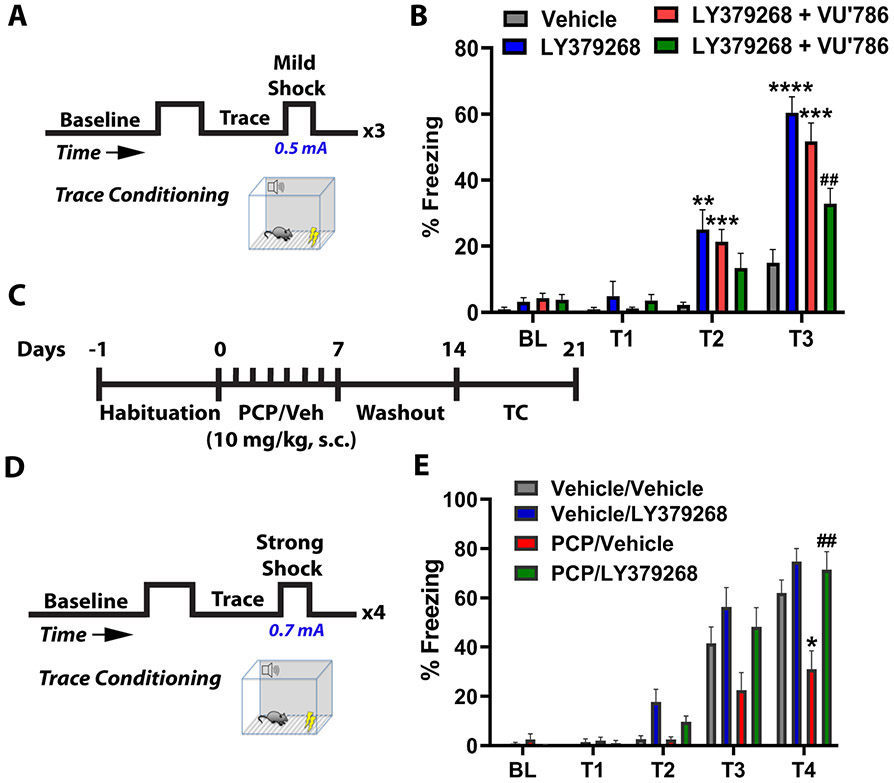
Figure 1. Activation of mGlu3 receptors enhances associative learning and rescues schizophrenia-like cognitive deficits.
(A) Behavioral schematic. Mice received 3 pairings of a tone and mild foot-shock (0.5 mA), each separated by a 30-second trace period. Freezing time was quantified across the 3 traces. (B) Administration of the mGlu2/3 agonist LY379268 (3 mg/kg, i.p., blue bars) 30 minutes prior to conditioning increased freezing at trace 2 and trace 3 relative to vehicle treated mice (grey bars) (**p<0.01, ****p<0.0001). Mice were treated with the mGlu2 negative allosteric modulator (NAM) VU6001966 (10 mg/kg, i.p., red bars) or the mGlu3 NAM VU0650786 (30 mg/kg, i.p., green bars) to isolate the effects of mGlu2 and mGlu3 receptor activation. LY379268 enhanced trace conditioning in VU6001966-treated mice (red bars, **p<0.01, ****p<0.0001 compared to vehicle (gray bars)), whereas the effect was blocked by the mGlu3 NAM VU0650786 (##p<0.01 compared to LY379268, F(3,72)=12.73 by two-way repeated measures ANOVA with Tukey’s post-hoc test, N=17-24 mice). (C) Schematic representing subchronic phencyclidine (scPCP) treatment regimen. After 7 days of habituation, mice were injected with scPCP (10 mg/kg) for 7 days. Trace fear conditioning experiments were performed 7 days after last PCP injection. (D) Behavioral schematic. Mice received 4 pairings of a tone, trace, and strong foot-shock (0.7 mA). (E) scPCP-treated mice (orange bars) froze less than vehicle controls (*p<0.05 compared to vehicle/vehicle; gray bars). Acute LY379268 treatment (30 minutes prior to the experiment; green bars) rescued scPCP-induced deficits in freezing (##p<0.01 compared to PCP/vehicle, red bars, F(3,35)=8.027 by two-way repeated measures ANOVA with Tukey’s post-hoc test, N=8-13). Data are presented as mean ± SEM.