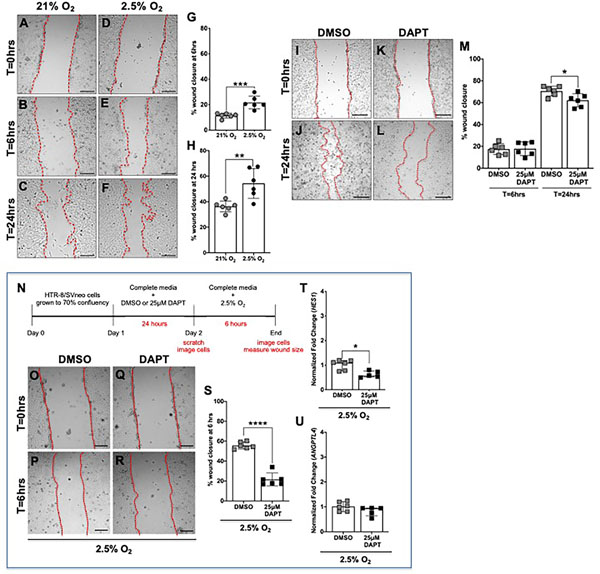
Figure 5. Hypoxia increases and inhibition of γ-secretase decreases HTR-8/Svneo migration in both normoxia and hypoxia.
(A-F) Representative images of the scratch migration assay at time (T) = 0 hours, T=6 hours, T=24 hours after exposure to normoxia (21% O2). or hypoxia (2.5% O2). HTR-8/SVneo cell migration was significantly increased with exposure to hypoxia for 6 hours (G) and 24 hours (H), (n = 6 per condition, **p <0.01, *** p<0.001). Data are represented as mean ± SD. (I-L) Representative images of the scratch migration assay at T=0 hours and T=24 hours after exposure to DMSO or 25μM DAPT. (M) HTR-8/SVneo cell migration was unchanged with exposure to 25μM DAPT for 6 hours and significantly decreased with exposure to 25μM DAPT for 24 hours in normoxic conditions (n = 6 per condition, *p <0.05). Data are represented as mean ± SD. (N) Timeline representing experimental design. HTR-8/SVneo cells were grown to 70% confluency and then DMSO or DAPT was added to the culture media. After 24 hours of exposure to 0.1% DMSO or 25μM DAPT, the treated media was removed and replaced with complete media. Scratches were made and cells were imaged at T=0 hours. HTR-8/SVneo cells were exposed to hypoxia (2.5% O2) for 6 hours and then imaged again. (O-R) Representative images of the scratch migration assay at time (T) =0 hours and T=6 hours. (S) Compared to pretreatment with DMSO, pretreatment with DAPT significantly decreased migration of cells exposed to hypoxic conditions (n = 6 per condition, ****p<0.0001). Data are represented as mean ± SD. (T, U) Expression of HES1 and ANGPTL4 in DAPT and DMSO pretreated cells was determined by qRT-PCR. HES1 (T) expression was significantly decreased and ANGPTL4 (U) was similar in HTR-8/SVneo cells that were pretreated with DAPT and then exposed to 2.5% O2 (n = 5–6 per condition, *p <0.05). Data are represented as median + IQR. Scale bars = 250 μm.