Abstract
Background
Patients with congenital heart disease (CHD) are at increased risk of developing ischemic stroke (IS) compared with controls without CHD. However, the long‐term outcomes after IS, including IS recurrence and mortality risk, remain unclear.
Methods and Results
We identified all patients with CHD in Sweden who were born between 1930 and 2017 using the Swedish National Patient Register and the Cause of Death Register. Ten controls without CHD were randomly selected from the general population and matched for birth year and sex for each patient with CHD. The follow‐up of the study population was performed between January 1970 and December 2017. In total, 88 700 patients with CHD (50.6% men) and 890 450 matched controls (51.0%) were included in this study. During a mean follow‐up of 25.1±22.0 years, patients with CHD had a 5‐fold higher risk of developing an index IS (hazard ratio [HR], 5.01; 95% CI, 4.81–5.22) compared with controls. However, the risk of developing a recurrent IS was lower in patients with CHD compared with controls (HR, 0.66; 95% CI, 0.56–0.78), an observation that persisted after adjustment for cardiovascular risk factors and comorbidities. Patients with CHD were also at a significantly lower risk of all‐cause mortality after index IS than controls (HR, 0.53; 95% CI, 0.49–0.58).
Conclusions
Patients with CHD had a 5‐fold higher risk of developing index IS compared with matched controls. However, the risk of recurrent IS stroke and all‐cause mortality were 34% and 47% lower, respectively, in patients with CHD compared with controls.
Keywords: congenital heart disease, ischemic stroke, mortality, recurrent stroke
Subject Categories: Congenital Heart Disease, Ischemic Stroke, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- IS
ischemic stroke
Clinical Perspective
What Is New?
The current study found a 5‐times increased risk of an ischemic stroke for patients with congenital heart disease when compared with matched controls.
The risk of recurrent ischemic stroke and mortality after ischemic stroke was lower among patients with congenital heart disease, most likely because of patients with congenital heart disease being younger and had fewer traditional cardiovascular risk factors when hospitalized with ischemic stroke compared with matched controls.
What Are the Clinical Implications?
The increased risk of stroke for patients with congenital heart disease must be considered when evaluating patients for cardiac interventions such as closure of a hemodynamically non‐significant right‐to‐left shunt or initiation of potent anticoagulation in a young patient with cardiac arrhythmias.
Congenital heart disease (CHD) affects at least 1% of all newborn children and is the most common major congenital anomaly.1, 2 Over the last century there have been immense advancements in the surgical and medical care of patients with CHD and today up to 97% of children with CHD reach age ≥18 years.3 The number of adults with CHD is currently larger than the number of children with CHD.4 Furthermore, patients with CHD are aging and the number of older patients with CHD is increasing.5, 6 Traditionally, the follow‐up of patients with CHD included management and treatment of CHD‐related complications, such as arrhythmias and heart failure.7, 8 However, with increasing age, patients with CHD are at risk of developing acquired cardiovascular diseases including myocardial infarction9, 10, 11 and ischemic stroke (IS).12, 13
IS is a serious condition causing significant morbidity and increased risk of premature mortality and other cardiovascular events including recurrent IS.14, 15 Compared with individuals without CHD, patients with CHD have an increased prevalence of heart failure and atrial arrhythmias,7, 8, 16, 17, 18, 19, 20 which are risk factors for IS as well as traditional cardiovascular risk factors. The risk of IS is increased in patients with CHD compared with controls.12, 21 However, the long‐term outcomes after IS in patients with CHD remain unknown.
The aims of the present study were to determine the cumulative incidence and risk of developing index IS, and to examine the risks of developing recurrent IS and all‐cause mortality after index IS, in patients with CHD compared with controls.
Methods
This study is based on data from the Swedish Inpatient Register held by the National Board of Health and Welfare (http://www.socialstyrelsen.se) and contains sensitive personal information. All data are subjected to secrecy in accordance with the Swedish Public Access to Information and Secrecy Act (OSL, 2009:400). The data set is coded and located at a secure server at the institution of the authors and is available given that ethical approval can be obtained for this purpose from the Swedish Ethical Review Authority at the University of Gothenburg. A formal request of the data can be made to corresponding author Dr Giang at Gothenburg University, Institution of Medicine, Department of Molecular and Clinical medicine (contact: wai.giang.kok@gu.se).
National Patient Registers
The present study is a nationwide study based on data from the Swedish Cause of Death Register and the Swedish National Patient Register, which are managed by the National Board of Health and Welfare. The National Patient Register was founded in 1964, had nationwide coverage since 1987, and contains both primary and all secondary discharge diagnoses and surgical procedures for all hospital admissions. Data on diagnoses in the hospital‐based outpatient clinics were also included in the National Patient Register since 2001. The International Classification of Diseases (ICD) system is used in the National Patient Register and the Cause of Death Register to code all diagnoses.
Study Population
A detailed description of the study population has previously been reported.3, 11 In brief, all patients born between 1930 and 2017 with a CHD diagnosis were identified in the National Patient Register and/or the Cause of Death Register. Each patient with CHD was matched by sex and birth year with ≈10 unique controls from the general population who did not have a CHD diagnosis during follow‐up time (identified from the Swedish Total Population Register). After being matched controls are not reused again for the next case. As such controls could only be matched once for each case of CHD.
Patients with CHD and controls were followed‐up from birth through the National Patient Register and the Cause of Death Register between 1970 and 2017. In the registers, a first‐time diagnosis of IS (defined as index IS) was identified, which included both non‐fatal and fatal IS diagnoses. Patients with CHD and controls who survived their index IS were further followed in the registers to determine any potential diagnosis of a recurrent fatal/non‐fatal IS and to assess all‐cause mortality.
In the current study a diagnosis of CHD could occur at the same date as IS which was the case for a total of 139 patients. However, since the follow‐up time was from birth a total of 29 people (22 controls and 7 CHD) were diagnosed with IS before or at their date of birth. Because of the uncertainty of these diagnoses of IS they were excluded. Patients with an atrial septal defect (ASD) or a patent foramen ovale share the same diagnostic code in the ICD, Eighth, Ninth and Tenth Revisions (ICD‐8, ICD‐9, ICD‐10), and therefore we excluded all patients diagnosed with an IS diagnosis and ASD/patent foramen ovale on the same date (n=835). This is because these patients were judged more likely to have a patent foramen ovale rather than an ASD. A separate sensitivity analysis with lesion group 6 excluding patent ductus arteriosus (n=8524) was performed since patent ductus arteriosus as an isolated lesion is in most cases benign. If it occurs in a premature child, it may even be considered a normal finding and should not be given a separate diagnosis. To avoid over‐including mild cases of CHD we excluded patent ductus arteriosus in this sensitivity analysis.
Definitions
The CHD diagnoses in the ICD‐8, ICD‐9, and ICD‐10 used to define the study population are shown in Table S1. We used a modified hierarchical classification system based on previous classifications.22, 23, 24 A patient with several CHD diagnoses is placed in the diagnostic group to which the most severe CHD diagnosis belongs. The 6 CHD diagnostic groups and corresponding diagnoses are presented in Table S2.
The CHD lesion group 1 included the diagnosis of conotruncal defects such as transposition of the great vessels, common arterial trunk, double‐outlet right ventricle, double‐outlet left ventricle, tetralogy of Fallot, discordant atrioventricular connection, and aortopulmonary septal defect. The CHD lesion group 2 included the diagnosis of severe non‐conotruncal defects such as hypoplastic left heart syndrome, endocardial cushion defects, and common ventricle. The CHD lesion group 3 included the diagnosis of coarctation of the aorta, the CHD lesion group 4 included the diagnosis of ventricular septal defect, and the CHD lesion group 5 included the diagnosis of ASD. The CHD lesion group 6 was defined as all other heart and circulatory system anomalies that were not included in the other CHD lesion groups.
The following ICD codes in any position were used to identify IS: ICD codes 433 or 434 in ICD‐8, ICD codes 434 or 436 in ICD‐9, and ICD codes I63 or I64 in ICD‐10. We defined the cardiovascular risk factors hypertension, diabetes mellitus, and hyperlipidemia as present if they were identified in the registers before the index IS, at time of index IS, or within 1 year from index IS. We defined the comorbidities atrial fibrillation, heart failure, and previous myocardial infarction as present if they were diagnosed before or simultaneously as index IS. Further details on the ICD codes of cardiovascular risk factors and comorbidities are presented in Table S3.
Recurrent IS was defined as a diagnosis of IS that occurred ≥1 month after the index IS. However, recurrent IS that was only identified in the outpatient register was not considered a recurrent event because these diagnoses likely represented the follow‐up visits for the index IS.
Statistical Analysis
All statistical analyses were performed with statistical software (R v3.5.2; R Foundation for Statistical Computing, Vienna, Austria). A P value <0.05 was considered statistically significant. For the index IS, categorical data are presented descriptively as numbers and percentages, while continuous data are presented as means and SD or medians and interquartile range (IQR). In the present study, both patients with CHD and controls were followed‐up from birth until event, death, or end of the study (December 31, 2017), whichever occurred first. The incidence rates were reported as the number of IS events per 10 000 person‐years, calculated as the number of IS events divided by the total follow‐up time of the population. The cumulative incidence during follow‐up with 95% CI of the index IS was estimated according to the Fine–Gray method (using R package prodlim). A competing event was death attributable to all causes other than IS. Hazard ratios (HR) with 95% CIs for the risk of developing index IS in cases and controls were obtained from Cox proportional‐hazard regression models. For all Cox analysis the reference group was the control population. In a separate analysis lesion group was tested as an effect modifier and the result was significant (P<0.001). Lesions were analyzed as all CHD followed by separate models for each lesion groups. Because of non‐proportionality because of the long follow‐up time for some of the regression models, time was divided into intervals of 0 to 17, 18 to 54, 55 to 64, 65 to 74, and ≥75 years and are presented in Table S4. All final models met the requirement of proportionality.
For recurrent IS and mortality after the index IS, the cases and controls who survived their index IS were further followed in the registers until a recurrent IS event, death, or the end of follow‐up (December 31, 2017), whichever occurred first. The cumulative incidence of a recurrent IS event was estimated separately for cases and controls according to the Fine–Grey method (using R package prodlim) with a 95% CI where death from causes other than IS were considered as a competing event. For all‐cause mortality after index IS, Kaplan–Meier functions with 95% CIs were performed to calculate the survival probability. HRs and CIs for the risk of developing recurrent IS and all‐cause mortality in cases and controls were obtained from Cox proportional‐hazard regression models. Data are presented unadjusted, model 1 (adjusted for age and sex at index IS, diabetes mellitus, hypertension, and hypercholesterolemia), and model 2 (adjusted for age and sex at index IS, diabetes mellitus, hypertension, hypercholesterolemia, atrial fibrillation, heart failure, and myocardial infarction). The follow‐up time was divided into time intervals of 0 to 4, 5 to 9, 10 to 14, and ≥15 years because of non‐proportionality because of long follow‐up time after index IS (Table S5 and S6). In addition, to meet the requirement of proportionality in the adjusted models some of the comorbidities were stratified in the Cox model. After stratification, all final models met the requirement of proportionality.
Ethical Approval
This study was approved by the regional ethics board in Gothenburg (Gbg 912–16, T 616–18). The Declaration of Helsinki was followed and the regional ethics board in Gothenburg waived the need for patient consent because the study was based on anonymized register‐based data. The Swedish National Board of Health and Welfare and Statistics Sweden performed identification of cases and controls and the linkage between the registers. The final data set that we received from the Swedish National Board of Health and Welfare contained pseudonymized data where the national personal identification numbers were replaced by a code.
Results
Baseline Characteristics of the Study Population
A total of 88 700 patients with CHD (50.6% men) and 890 450 controls (51.0% men) without CHD who were born between 1930 and 2017 were included in the present study. The majority of patients with CHD were born between 1990 and 2017 (n=54 543, 61.5%), while patients born in 1930 to 1969 and 1970 to 1989 represented ≈19.1% and 19.4%, respectively, of the study population. The mean follow‐up time was 25.1±22.0 years in patients with CHD and 27.3±22.0 years in controls. The baseline characteristics of the study population are shown in Table 1.
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | Congenital Heart Disease, n=88 700 (%) | Controls, n=890 450 (%) |
|---|---|---|
| Sex | ||
| Male | 44 903 (50.6%) | 453 704 (51.0%) |
| Female | 43 797 (49.4%) | 436 746 (49.0%) |
| Birth year±SD | 1990.2±22.5 | 1989.8±22.6 |
| Birth period, y | ||
| 1930‒1969 | 16 965 (19.1%) | 176 010 (19.8%) |
| 1970‒1989 | 17 192 (19.4%) | 173 590 (19.5%) |
| 1990‒2017 | 54 543 (61.5%) | 540 850 (60.7%) |
| CHD lesion group | ||
| Lesion group 1* | 6326 (7.1%) | 63 040 (7.1%) |
| Lesion group 2† | 4831 (5.4%) | 48 199 (5.4%) |
| Lesion group 3‡ | 4599 (5.2%) | 45 868 (5.2%) |
| Lesion group 4§ | 25 150 (28.4%) | 249 311 (28.0%) |
| Lesion group 5|| | 20 089 (22.6%) | 208 086 (23.4%) |
| Lesion group 6# | 27 705 (31.2%) | 275 946 (31.0%) |
| Born in Sweden | ||
| Yes | 82 379 (92.9%) | 719 510 (80.8%) |
| No | 6321 (7.1%) | 170 940 (19.2%) |
| Index IS | 3187 (3.6%) | 7348 (0.82%) |
| Fatal index IS | 70 (2.2%) | 320 (4.3%) |
Fatal index IS percentage is based on number of index IS.
CHD indicates congenital heart disease; and IS, ischemic stroke.
Lesion group 1 was defined as conotruncal defects (aortopulmonary septal defect, common arterial trunk, tetralogy of Fallot, transposition of the great arteries [unrepaired lesions and surgically repaired], double‐outlet right ventricle, double‐outlet left ventricle, congenitally corrected transposition/discordant atrioventricular, and ventriculoatrial connection).
Lesion group 2 was defined as severe non‐conotruncal defects (endocardial cushion defect/atrioventricular septal defect, common ventricle, and hypoplastic left heart syndrome). This group contains univentricular heart defects.
Lesion group 3 was defined as coarctation of the aorta.
Lesion group 4 was defined as ventricular septal defect.
Lesion group 5 was defined as atrial septal defect.
Lesion group 6 was defined as all other heart and circulatory system anomalies that were not included in the other lesion groups.
Index IS
The cumulative incidence of IS at age 20, 50, and 75 years was 0.4%, 5.6%, and 20.8%, respectively, in patients with CHD compared with 0.02%, 0.4%, and 6.8%, respectively, in controls. The cumulative incidence of IS was low in patients with CHD and controls until ≈20 years old. However, the cumulative incidence of IS increased more rapidly in patients with CHD compared with controls (Figure 1). The cumulative incidence of IS in the 6 CHD lesion groups is shown in Figure 2.
Figure 1. The cumulative incidence of IS stroke in patients with CHD compared with controls.
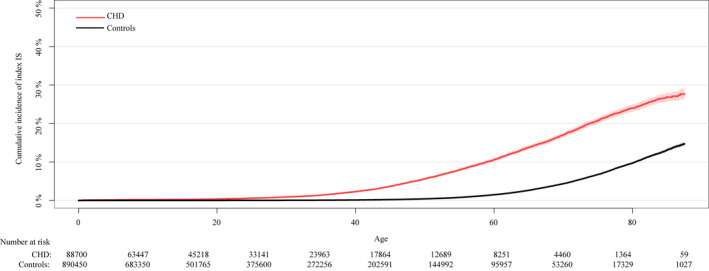
CHD indicates congenital heart disease; and IS, ischemic stroke.
Figure 2. The cumulative incidence of index IS according to the 6 different CHD lesion groups.
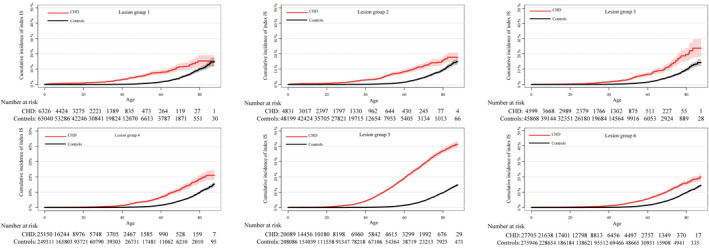
CHD indicates congenital heart disease; and IS, ischemic stroke.
Patients with CHD who developed an index IS were markedly younger than their matched controls that developed an index IS; the median age for patients with CHD was 52.1 (IQR, 39.8–63.6) years versus 66.0 (IQR, 57.1–73.4) years for controls (P<0.001). Patients with ASD diagnosis (CHD lesion group 5) had the highest incidence rate of IS (31.3 cases per 10 000 patient years), while patients with ventricular septal defect (CHD lesion group 4) had the lowest incidence rate of IS (6.6 cases per 10 000 patient‐years) (Table 2).
Table 2.
Incidence Rates of Ischemic Stroke in Patients With Congenital Heart Disease and Controls
| Congenital Heart Disease | Controls | |||||
|---|---|---|---|---|---|---|
| Lesion Group | Total Number of Patients | Ischemic Stroke, n (%) | Incidence Rate Per 10 000 Patient‐Years (95% CI) | Total Number of Patients | Ischemic Stroke, n (%) | Incidence Rate Per 10 000 Patient‐Years (95% CI) |
| All CHD | 88 700 | 3257 (3.7) | 14.82 (14.31–15.33) | 890 450 | 7668 (0.9) | 3.16 (3.09–3.23) |
| Lesion group 1* | 6326 | 169 (2.7) | 12.23 (10.45–14.21) | 63 040 | 312 (0.5) | 1.77 (1.58–1.97) |
| Lesion group 2† | 4831 | 196 (4.1) | 17.58 (15.21–20.22) | 48 199 | 451 (0.9) | 2.87 (2.61–3.15) |
| Lesion group 3‡ | 4599 | 145 (3.2) | 10.43 (8.80–12.28) | 45 868 | 452 (1.0) | 2.97 (2.70–3.26) |
| Lesion group 4§ | 25 150 | 301 (1.2) | 6.58 (5.86–7.37) | 249 311 | 947 (0.4) | 1.99 (1.87–2.13) |
| Lesion group 5|| | 20 089 | 1796 (8.9) | 31.25 (29.82–37.72) | 208 086 | 3126 (1.5) | 4.92 (4.75–5.10) |
| Lesion group 6# | 27 705 | 650 (2.3) | 8.36 (7.73–9.03) | 275 946 | 2380 (0.9) | 2.86 (2.74–2.97) |
CHD indicates congenital heart disease.
Lesion group 1 was defined as conotruncal defects (aortopulmonary septal defect, common arterial trunk, tetralogy of Fallot, transposition of the great arteries [unrepaired lesions and surgically repaired], double‐outlet right ventricle, double‐outlet left ventricle, congenitally corrected transposition/discordant atrioventricular, and ventriculoatrial connection).
Lesion group 2 was defined as severe non‐conotruncal defects (endocardial cushion defect/atrioventricular septal defect, common ventricle, and hypoplastic left heart syndrome). This group contains univentricular heart defects.
Lesion group 3 was defined as coarctation of the aorta.
Lesion group 4 was defined as ventricular septal defect.
Lesion group 5 was defined as atrial septal defect.
Lesion group 6 was defined as all other heart and circulatory system anomalies that were not included in the other lesion groups.
Patients with CHD had a 5‐fold increased risk of developing IS compared with controls without CHD (HR, 5.01; 95% CI, 4.81–5.22). Compared with controls, patients with conotruncal defects (CHD lesion group (1), severe non‐conotruncal defects (CHD lesion group (2), and ASD had the highest risk of developing IS, while the risks were similar for patients in the other CHD lesion groups (Table 3). Additionally, the risk of developing IS overall and by CHD lesion groups was highest during the first years of follow‐up, while differences in the risk for cases and controls decreased over time (Table S4). A sensitivity analysis excluding patent ductus arteriosus diagnosis in lesion group 6 showed similar results (Table S7). A sub‐analysis of patients born 1990 to 2017 showed an overall incidence rate of 3.64 and 0.23 per 10 000 person‐years for CHD and controls and with an increased risk of nearly 16 times when compared with controls (Table S8 and S9).
Table 3.
HRs for Index Ischemic Stroke in Patients with CHD Compared With Controls
| Lesion Group | No. of Events CHD/Controls | HR (95% CI) |
|---|---|---|
| All CHD | 3257/7668 | 5.01 (4.81–5.22) |
| Lesion group 1* | 169/312 | 7.99 (6.62–9.64) |
| Lesion group 2† | 196/451 | 5.63 (4.76–6.66) |
| Lesion group 3‡ | 145/452 | 4.00 (3.31–4.82) |
| Lesion group 4§ | 301/947 | 3.63 (3.18–4.13) |
| Lesion group 5|| | 1796/3126 | 6.73 (6.35–7.13) |
| Lesion group 6# | 650/2380 | 3.14 (2.88–3.42) |
Control population as reference group.
CHD indicates congenital heart disease; and HR, hazard ratio.
Lesion group 1 was defined as conotruncal defects (aortopulmonary septal defect, common arterial trunk, tetralogy of Fallot, transposition of the great arteries [unrepaired lesions and surgically repaired], double‐outlet right ventricle, double‐outlet left ventricle, congenitally corrected transposition/discordant atrioventricular, and ventriculoatrial connection).
Lesion group 2 was defined as severe non‐conotruncal defects (endocardial cushion defect/atrioventricular septal defect, common ventricle, and hypoplastic left heart syndrome). This group contains univentricular heart defects.
Lesion group 3 was defined as coarctation of the aorta.
Lesion group 4 was defined as ventricular septal defect.
Lesion group 5 was defined as atrial septal defect.
Lesion group 6 was defined as all other heart and circulatory system anomalies that were not included in the other lesion groups.
Baseline Characteristics of the Study Population with Index IS
A total of 70 patients with CHD and 320 controls had a fatal index IS (Table 1). Altogether 3187 patients with CHD (55.9% men) and 7348 controls (57.9% men) who survived an index IS were followed‐up further in the registers and evaluated for the risk of recurrent IS (fatal or non‐fatal) and all‐cause mortality. The demographic characteristics, cardiovascular risk factors, and comorbidities in this population are shown in Table 4. The mean follow‐up time after the index IS was 8.4±7.0 years in patients with CHD and 7.0±6.4 years in controls.
Table 4.
Demographic Characteristics, Cardiovascular Risk Factors, and Comorbidities in Patients with CHD and Controls Who Survived Index Ischemic Stroke and Were Further Followed in the Registers
| Congenital Heart Disease, n=3187 (%) | Controls, n=7348 (%) | |
|---|---|---|
| Sex | ||
| Male | 1782 (55.9) | 4255 (57.9) |
| Female | 1405 (44.1) | 3093 (42.1) |
| CHD lesion group | ||
| Lesion group 1* | 155 (4.9) | 306 (4.2) |
| Lesion group 2† | 191 (6.0) | 427 (5.8) |
| Lesion group 3‡ | 137 (4.3) | 437 (5.9) |
| Lesion group 4§ | 296 (9.3) | 906 (12.3) |
| Lesion group 5|| | 1784 (56.0) | 2995 (40.8) |
| Lesion group 6# | 624 (19.6) | 2277 (31.0) |
| Birth period, y | ||
| 1930‒1959 | 2501 (78.5) | 6954 (94.6) |
| 1960‒1989 | 467 (14.7) | 252 (3.4) |
| 1990‒2017 | 219 (6.9) | 142 (1.9) |
| Hypertension | 959 (30.1) | 3817 (51.9) |
| Diabetes mellitus | 294 (9.2) | 1475 (20.1) |
| Hyperlipidemia | 517 (16.2) | 1486 (20.2) |
| Atrial fibrillation | 443 (13.9) | 729 (9.9) |
| Heart failure | 333 (10.4) | 508 (6.9) |
| Myocardial infarction | 169 (5.3) | 676 (9.2) |
CHD indicates congenital heart disease.
Lesion group 1 was defined as conotruncal defects (aortopulmonary septal defect, common arterial trunk, tetralogy of Fallot, transposition of the great arteries [unrepaired lesions and surgically repaired], double‐outlet right ventricle, double‐outlet left ventricle, congenitally corrected transposition/discordant atrioventricular, and ventriculoatrial connection).
Lesion group 2 was defined as severe non‐conotruncal defects (endocardial cushion defect/atrioventricular septal defect, common ventricle, and hypoplastic left heart syndrome). This group contains univentricular heart defects.
Lesion group 3 was defined as coarctation of the aorta.
Lesion group 4 was defined as ventricular septal defect.
Lesion group 5 was defined as atrial septal defect.
Lesion group 6 was defined as all other heart and circulatory system anomalies that were not included in the other lesion groups.
Long‐Term Risk of Recurrent IS
Patients with CHD had a lower risk of developing a recurrent IS compared with controls (Figure 3). At 5 and 20 years of follow‐up, the cumulative incidence of a recurrent IS was 2.8% and 11.4%, respectively, in patients with CHD compared with 4.1% and 13.0%, respectively, in controls. As for the index IS, patients with CHD were younger than controls at recurrent IS; the median age at recurrent IS was 61.6 (IQR, 50.3–71.6) years in patients with CHD compared with 71.0 (IQR, 65.0–77.0) years in controls (P<0.001).
Figure 3. The cumulative incidence of recurrent IS in patients with CHD compared with controls.
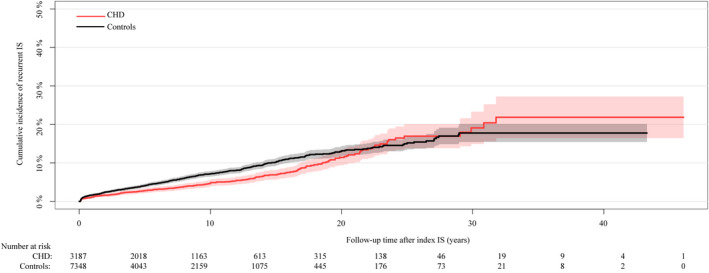
CHD indicates congenital heart disease; and IS, ischemic stroke.
In a Cox‐regression analysis, the risk of patients with CHD developing recurrent IS was 34% lower than the risk in controls (HR, 0.66; 95% CI, 0.56–0.78). This lower risk in patients with CHD appeared to be mainly driven by a lower risk of developing recurrent IS in patients with ASD (CHD lesion group 5) (Table 5). This lower recurrent IS risk in the CHD group compared with controls persisted after adjustment for age at index IS, sex, hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, heart failure, and myocardial infarction (model 2: HR, 0.82; 95% CI, 0.68–0.98) (Table 5). The risk of recurrent IS by time intervals showed no differences in risk between cases and controls during the first years (0–4 years: HR, 0.81; 95% CI, 0.63–1.06) of follow‐up. However, patients with CHD had a lower risk at 5–9 years (HR, 0.62; 95% CI, 0.42–0.90). Thereafter there were no differences in the risk of recurrent IS between cases and controls (Table S5). Overall, there were no differences in the risk by time intervals for CHD lesions groups except for lesion group 5 during the first 10 years, with an overall lower risk of recurrent IS (0–4 years: HR, 0.60; 95% CI, 0.42–0.87; 5–9 years: HR, 0.40; 95% CI, 0.24–0.67).
Table 5.
Risk of Recurrent Ischemic Stroke in Patients with CHD Compared With Controls
| No. of Recurrent Events, CHD/Controls | HR (95% CI), Unadjusted | HR (95% CI), Adjusted, Model 1 | HR (95% CI), Adjusted, Model 2 | |
|---|---|---|---|---|
| All CHD | 188/539 | 0.66 (0.56–0.78) | 0.89 (0.74–1.05) | 0.82 (0.68–0.98) |
| Lesion group 1* | 10/24 | 0.71 (0.34–1.50) | 1.28 (0.53–3.09) | 1.49 (0.58–3.78) |
| Lesion group 2† | 10/33 | 0.49 (0.24–1.00) | 0.55 (0.26–1.18) | 0.45 (0.20–1.00) |
| Lesion group 3‡ | 8/26 | 0.68 (0.30–1.54) | 0.80 (0.34–1.89) | 0.58 (0.23–1.41) |
| Lesion group 4§ | 14/55 | 0.67 (0.37–1.21) | 1.10 (0.59–2.04) | 1.13 (0.60–2.13) |
| Lesion group 5|| | 107/254 | 0.56 (0.45–0.71) | 0.74 (0.58–0.95) | 0.68 (0.53–0.87) |
| Lesion group 6# | 39/147 | 0.87 (0.61–1.24) | 1.21 (0.84–1.75) | 1.12 (0.77–1.63) |
Control population as reference group.
Model 1: Adjusted for age at index ischemic stroke, sex, hypertension, diabetes mellitus, and hyperlipidemia.
Model 2: Adjusted for age at index ischemic stroke, sex, hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, heart failure, and myocardial infarction.
CHD indicates congenital heart disease; and HR, hazard ratio.
Lesion group 1 was defined as conotruncal defects (aortopulmonary septal defect, common arterial trunk, tetralogy of Fallot, transposition of the great arteries [unrepaired lesions and surgically repaired], double‐outlet right ventricle, double‐outlet left ventricle, congenitally corrected transposition/discordant atrioventricular, and ventriculoatrial connection).
Lesion group 2 was defined as severe non‐conotruncal defects (endocardial cushion defect/atrioventricular septal defect, common ventricle, and hypoplastic left heart syndrome). This group contains univentricular heart defects.
Lesion group 3 was defined as coarctation of the aorta.
Lesion group 4 was defined as ventricular septal defect.
Lesion group 5 was defined as atrial septal defect.
Lesion group 6 was defined as all other heart and circulatory system anomalies that were not included in the other lesion groups.
Long‐Term Risk of All‐Cause Mortality After Index IS
The cumulative incidence of all‐cause mortality at 5 and 20 years after the index IS was lower in patients with CHD compared with controls (Figure 4, CHD: 14.2% and 41.7%, respectively; controls: 25.8% and 64.2%, respectively). Nevertheless, patients with CHD who died after index IS were younger compared with controls (median age at death of 67.9 [IQR, 53.7–76.6] years for CHD compared with 73.0 [IQR, 66.1–78.6] years for controls; P<0.001).
Figure 4. Kapan−Meier curve illustrating survival probability after IS in patients with CHD compared with controls.
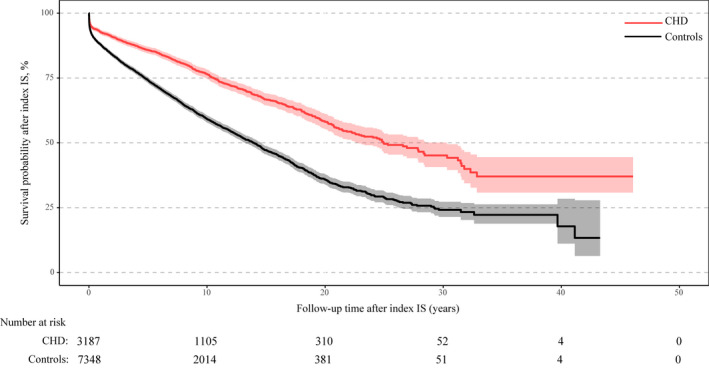
CHD indicates congenital heart disease; and IS, ischemic stroke.
The risk of all‐cause mortality in patients with CHD was almost half that of controls (HR, 0.53; 95% CI, 0.49–0.58). This lower risk of mortality persisted even after adjustment for age at index IS, hypertension, diabetes mellitus, and hyperlipidemia, as well as for atrial fibrillation, heart failure, and previous myocardial infarction (Table 6). However, results were directionally opposite depending on the CHD lesion group. Patients with ASD (CHD lesion group 5) had a lower risk of all‐cause mortality after index IS compared with controls, which persisted despite adjustments for age at index IS, after adjustments for age at IS, hypertension, diabetes mellitus, hyperlipidemia (model 1), and after additional adjustment for atrial fibrillation, heart failure, and previous myocardial infarction (model 2). Patients with severe conotruncal defects (CHD lesion group 1) had a 2‐fold higher risk of mortality compared with controls after index IS (Table 6).
Table 6.
Risk of Mortality After Ischemic Stroke in Patients with CHD Compared With Controls
| No. of Deaths, CHD/Controls | HR (95% CI), Unadjusted | HR (95% CI), Adjusted, Model 1 | HR (95% CI), Adjusted, Model 2 | |
|---|---|---|---|---|
| All CHD | 801/2919 | 0.53 (0.49–0.58) | 0.83 (0.77–0.90) | 0.74 (0.68–0.81) |
| Lesion group 1* | 65/105 | 1.12 (0.82–1.53) | 2.39 (1.63–3.49) | 1.85 (1.20–2.84) |
| Lesion group 2† | 74/181 | 0.73 (0.56–0.96) | 1.02 (0.76–1.37) | 0.88 (0.64–1.20) |
| Lesion group 3‡ | 56/158 | 0.88 (0.64–1.20) | 1.32 (0.95–1.83) | 1.09 (0.77–1.54) |
| Lesion group 4§ | 91/323 | 0.78 (0.62–0.98) | 1.21 (0.95–1.54) | 1.05 (0.82–1.35) |
| Lesion group 5|| | 317/1280 | 0.34 (0.30–0.38) | 0.60 (0.53–0.69) | 0.55 (0.48–0.62) |
| Lesion group 6# | 198/872 | 0.76 (0.65–0.89) | 1.14 (0.97–1.34) | 1.01 (0.86–1.19) |
Control population as reference group.
Model 1: Adjusted for age at index ischemic stroke, sex, hypertension, diabetes mellitus, and hyperlipidemia.
Model 2: Adjusted for age at index ischemic stroke, sex, hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, heart failure, and myocardial infarction.
CHD indicates congenital heart disease; and HR, hazard ratio.
Lesion group 1 was defined as conotruncal defects (aortopulmonary septal defect, common arterial trunk, tetralogy of Fallot, transposition of the great arteries [unrepaired lesions and surgically repaired], double‐outlet right ventricle, double‐outlet left ventricle, congenitally corrected transposition/discordant atrioventricular, and ventriculoatrial connection).
Lesion group 2 was defined as severe non‐conotruncal defects (endocardial cushion defect/atrioventricular septal defect, common ventricle, and hypoplastic left heart syndrome). This group contains univentricular heart defects.
Lesion group 3 was defined as coarctation of the aorta.
Lesion group 4 was defined as ventricular septal defect.
Lesion group 5 was defined as atrial septal defect.
Lesion group 6 was defined as all other heart and circulatory system anomalies that were not included in the other lesion groups.
For the time intervals, patients with CHD had an overall lower risk of death after index IS (Table S6). Differences in risk over time were observed for the different lesion groups. The highest risk was observed in lesion group 1 (HR, 2.39; 95% CI, 1.42–4.03) during the first years after index IS. However, the increased risk decreased over time and there was no significant difference at end of the study (HR, 1.61; 95% CI, 0.57–4.59). No risk differences were observed during the first years of follow‐up for the other CHD lesion groups except for lesion group 5, where CHD had an overall lower risk of mortality after index IS.
Discussion
In this large, nationwide, register‐based study that included 88 700 patients with CHD and 890 450 age‐ and sex‐matched controls, the risk of index IS was 5‐fold higher in patients with CHD compared with controls. The risk was predominantly observed in patients with the most complex lesions, lesion groups 1 and 2, and in patients with ASD. Overall, the risk of developing a recurrent IS after an index event was lower in patients with CHD compared with controls. This result remained after adjusting for age at index IS and for cardiovascular comorbidities (hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, heart failure, and previous myocardial infarction). Furthermore, the risk of all‐cause mortality after index IS in patients with CHD was almost half of that in control subjects (HR, 0.53; 95% CI, 0.49–0.58).
Our findings on the risk of index IS are supported by a study by Pedersen et al, who used data from the Danish Patient Register to study the risk of IS in adult patients with CHD.12 Although that study reported a somewhat lower cumulative incidence of first time IS in CHD patients compared with the present study, the risks of IS were comparable—Pedersen et al reported a 3.8‐fold increased risk of IS in patients with CHD aged <60 years (HR, 3.8; 95% CI, 3.3–4.3) and a 1.6‐fold increased risk in patients with CHD aged >60 years (HR, 1.6; 95% CI, 1.4–1.9).12 However, unlike the present study, the study by Pedersen et al only included individuals aged >18 years. Similar trends to our findings were also reported by Lanz et al, who found a 9‐ to 12‐fold higher risk of IS in patients with CHD aged <55 years compared with the general population and a 2‐ to 4‐fold higher risk in patients with CHD aged 55 to 64 years.13 In that study, the predictors of IS included heart failure, diabetes mellitus, and recent myocardial infarction. Furthermore, in a study of younger patients with CHD, we reported an ≈10‐fold increased risk of developing IS compared with controls.21 In our study, we found a nearly 16 times increased risk of IS among younger patients with CHD in the sub‐analysis of patients born in 1990 to 2017.
There are several potential explanations for the increased risk of IS in patients with CHD. First, compared with patients without CHD, patients with CHD show a higher burden of atrial fibrillation and heart failure,7, 8, 20, 25 which are both recognized risk factors for IS. Second, patients with CHD have a relatively high prevalence of mechanical heart valves, which also increases the risk of embolic IS, while a significant proportion of patients with CHD have undergone surgical/interventional procedures that may lead to perioperative IS. Third, inter‐atrial communication through an ASD, ventricular septal defect, or arterial‐venous shunt, for example, also increases the risk of IS via paradoxical emboli. Fourth, hyper‐viscosity states that can be associated with some CHD lesions may increase the risk for IS.26 Finally, traditional cardiovascular risk factors such as hypertension, diabetes mellitus, and hyperlipidemia, as well as smoking, also contribute to IS in patients with CHD, while some cardiovascular risk factors including diabetes mellitus are more common in patients with CHD than in controls, and in addition, treatment with extracorporeal membrane oxygenation and ventricular assist devices (VADs) early in life could also contribute to an increased risk of IS.27, 28, 29
To our knowledge, the present study is the first to present the risks of recurrent IS and all‐cause mortality in patients with CHD after an IS. We found that the risk of recurrent IS was markedly lower in patients with CHD compared with controls, which was largely driven by a decreased risk of recurrent IS events in patients with ASD. Of note, adjusting for traditional cardiovascular risk factors (e.g., hypertension, hyperlipidemia, and diabetes mellitus) and for atrial fibrillation, heart failure, and myocardial infarction did not change this observation of a lower risk for recurrent IS in patients with CHD. This likely reflects the somewhat different mechanisms of IS in patients with CHD compared with controls. Patients with CHD with an index IS had markedly less hypertension, hyperlipidemia, and diabetes mellitus compared with controls with an index IS. However, patients with CHD had a higher incidence rate of index IS. It is plausible that the predominantly ‘CHD‐related’ risk factors for IS (eg, atrial fibrillation, heart failure, and mechanical valves) contribute less to a recurrent IS or death than for traditional cardiovascular risk factors, which is reflected in a lower incidence of composite events in patients with CHD compared with controls. Furthermore, it is possible that some of the IS in our population was related to potentially modifiable causes (e.g., residual shunts), which after intervention, contributed less to causing recurrent IS. The latter is reflected in the markedly lower risk of recurrent events in patients with ASDs—potential closure of right‐to‐left shunts may explain the markedly lower risk for recurrent events in the ASD group. In addition, differences in medical treatments of stroke among patients with CHD with IS may differ from controls especially in the use of antithrombotic agents such as anticoagulation or antithrombotic treatment over time.
In the present study, the risk of mortality after index IS was almost halved in patients with CHD compared with controls. Furthermore, the mortality was largely driven by a lower mortality risk in patients with ASDs, who also represented approximately half of the population with index IS. The lower mortality risk in patients with ASD may reflect the differences in the mechanisms of IS in patients with ASDs (e.g., right‐left interatrial communications, atrial fibrillation) compared with controls, who had more traditional cardiovascular risk factors such as hypertension and diabetes mellitus. Patients with CHD were also younger than controls at the age of index IS, which likely reflects the different stroke mechanisms in patients with CHD. Additionally, the number of fatal indexes IS differed slightly between patients with CHD and controls. However, since the mortality was low, 2% and 4% respectively, the effect of this survival bias will be modest.
Another explanation for the lower mortality rates in patients with CHD may relate to the younger age at index IS of patients with CHD—the differences in the risks of mortality between patients with CHD and controls were no longer seen after adjustment for age at index IS for all diagnostic groups except ASDs and severe conotruncal defects. By contrast, the risk of mortality was increased in patients with conotruncal defects after adjustments for age and comorbidities, which likely reflects the severity of these lesions and the increased mortality risks they carry.
Strengths and Limitations
One of the main strengths of the present study was the large number of included patients with minimal loss of follow‐up. We were also able to match almost every individual with 10 controls from the general population. Furthermore, both young and old patients were included, which provides information on the CHD population as a group. To the best of our knowledge, this is the first report of long‐term outcomes after IS in a nationwide and large population of patients with CHD.
A limitation of this study is the potential for errors because of miscoding of the diagnoses, as for all register‐based studies. However, internal (with medical records) and external validation (with other registers) studies, based on diagnoses in the Swedish National Patient Register, found that the positive predictive value was 85% to 95% for most of the cardiovascular diagnoses.30 In addition, validation of stroke diagnosis specifically for younger children in the registers have not been previously done. However, children with diagnosed stroke are most likely closely followed‐up by pediatrician and closely monitored and as such the validity of the diagnoses could be considered to be of equal quality among patients with CHD and controls. Although there are limited data on the validity of various diagnoses in the National Patient Register in patients with CHD, a validation study by our group concluded that the validity of myocardial infarction diagnoses in patients with CHD from the National Patient Register was high.31 However, because of the register‐based design of the present study, there is likely to be some misclassification of CHD diagnoses and incorrectly coded diagnoses.
Our study included patients with CHD of all ages who were born between 1930 and 2017. However, as the follow‐up in the registers started in 1970, we have no data on potential IS cases that occurred between 1930 and 1969. Thus, there is potential for a survivorship bias. We also do not have detailed clinical information on stroke severity in patients with CHD and controls or information on the frequency of perioperative IS. Detailed clinical data on cardiovascular risk factors including blood pressure levels, cholesterol levels, and smoking were also unavailable. Some cardiovascular risk factors may have been omitted in the registers as the cardiovascular risk factors of many patients are assessed in primary care. Additionally, we have no data collected during heart failure or atrial fibrillation or any information on potential anticoagulation treatments of the patients. After index IS the matching was not maintained because the aim of the study was to follow CHD and controls from birth until end of study, reflecting a life course perspective of the study population. As such after index IS there were some differences in age, sex, and cardiovascular risk factors (Table S8). These were adjusted for in the models for recurrent IS and mortality. Finally, because of the long follow‐up of our study, there were likely changes in the diagnosis of IS cases related to the widespread use of computed tomography and magnetic resonance imaging. This may have resulted in identification and registration of a higher proportion of milder IS cases in the more contemporary period of the study.
Conclusions
Patients with CHD had an increased risk of developing index IS compared with controls. However, the risk of developing a recurrent IS was one‐third lower in patients with CHD compared with controls, while the risk of all‐cause mortality after index IS was almost halved in patients with CHD compared with controls. The causes of IS and the resulting risk of consecutive events may differ between patients with and without CHD.
Sources of Funding
The study was financed by grants from the Swedish state under the agreement between the Swedish government and the country councils, the ALF‐agreement (grant numbers, 917361 and 236611), and the Swedish Heart‐Lung Foundation (grant numbers, 20090724 and 20180644).
Disclosures
None.
Supporting information
Tables S1–S9
(J Am Heart Assoc. 2021;10:e020939. DOI: 10.1161/JAHA.120.020939.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020939
For Sources of Funding and Disclosures, see page 11.
References
- 1.Khoshnood B, Lelong N, Houyel L, Thieulin A‐C, Jouannic J‐M, Magnier S, Delezoide A‐L, Magny J‐F, Rambaud C, Bonnet D, et al. Prevalence, timing of diagnosis and mortality of newborns with congenital heart defects: a population‐based study. Heart (British Cardiac Society). 2012;98:1667–1673. DOI: 10.1136/heartjnl-2012-302543. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Chen S, Zuhlke L, Black GC, Choy MK, Li N, Keavney BD. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta‐analysis of 260 studies. Int J Epidemiol. 2019;48:455–463. DOI: 10.1093/ije/dyz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandalenakis Z, Giang KW, Eriksson P, Liden H, Synnergren M, Wåhlander H, Fedchenko M, Rosengren A, Dellborg M. Survival in children with congenital heart disease: have we reached a peak at 97%? J Am Heart Assoc. 2020;9:e017704. DOI: 10.1161/JAHA.120.017704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marelli AJ, Mackie AS, Ionescu‐Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. DOI: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 5.Afilalo J, Therrien J, Pilote L, Ionescu‐Ittu R, Martucci G, Marelli AJ. Geriatric congenital heart disease: burden of disease and predictors of mortality. J Am Coll Cardiol. 2011;58:1509–1515. DOI: 10.1016/j.jacc.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 6.Tutarel O, Kempny A, Alonso‐Gonzalez R, Jabbour R, Li W, Uebing A, Dimopoulos K, Swan L, Gatzoulis MA, Diller GP. Congenital heart disease beyond the age of 60: emergence of a new population with high resource utilization, high morbidity, and high mortality. Eur Heart J. 2014;35:725–732. DOI: 10.1093/eurheartj/eht257. [DOI] [PubMed] [Google Scholar]
- 7.Norozi K, Wessel A, Alpers V, Arnhold JO, Geyer S, Zoege M, Buchhorn R. Incidence and risk distribution of heart failure in adolescents and adults with congenital heart disease after cardiac surgery. Am J Cardiol. 2006;97:1238–1243. DOI: 10.1016/j.amjcard.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 8.Bouchardy J, Therrien J, Pilote L, Ionescu‐Ittu R, Martucci G, Bottega N, Marelli AJ. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679–1686. DOI: 10.1161/CIRCULATIONAHA.109.866319. [DOI] [PubMed] [Google Scholar]
- 9.Olsen M, Marino B, Kaltman J, Laursen H, Jakobsen L, Mahle W, Pearson G, Madsen N. Myocardial infarction in adults with congenital heart disease. Am J Cardiol. 2017;120:2272–2277. DOI: 10.1016/j.amjcard.2017.08.050. [DOI] [PubMed] [Google Scholar]
- 10.Saha P, Potiny P, Rigdon J, Morello M, Tcheandjieu C, Romfh A, Fernandes SM, McElhinney DB, Bernstein D, Lui GK, et al. Substantial cardiovascular morbidity in adults with lower‐complexity congenital heart disease. Circulation. 2019;139:1889–1899. DOI: 10.1161/CIRCULATIONAHA.118.037064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fedchenko M, Mandalenakis Z, Giang KW, Rosengren A, Eriksson P, Dellborg M. Long‐term outcomes after myocardial infarction in middle‐aged and older patients with congenital heart disease‐a nationwide study. Eur Heart J. 2020;ehaa874. DOI: 10.1093/eurheartj/ehaa874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedersen MGB, Olsen MS, Schmidt M, Johnsen SP, Learn C, Laursen HB, Madsen NL. Ischemic stroke in adults with congenital heart disease: a population‐based cohort study. J Am Heart Assoc. 2019;8:e011870. DOI: 10.1161/JAHA.118.011870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanz J, Brophy JM, Therrien J, Kaouache M, Guo L, Marelli AJ. Stroke in adults with congenital heart disease: incidence, cumulative risk, and predictors. Circulation. 2015;132:2385–2394. DOI: 10.1161/CIRCULATIONAHA.115.011241. [DOI] [PubMed] [Google Scholar]
- 14.Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Anderson CS. Long‐term disability after first‐ever stroke and related prognostic factors in the Perth Community Stroke Study, 1989–1990. Stroke. 2002;33:1034–1040. DOI: 10.1161/01.STR.0000012515.66889.24. [DOI] [PubMed] [Google Scholar]
- 15.Boulanger M, Béjot Y, Rothwell PM, Touzé E. Long‐term risk of myocardial infarction compared to recurrent stroke after transient ischemic attack and ischemic stroke: systematic review and meta‐analysis. J Am Heart Assoc. 2018;7:e007267. DOI: 10.1161/JAHA.117.007267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piran S, Veldtman G, Siu S, Webb GD, Liu PP. Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation. 2002;105:1189–1194. DOI: 10.1161/hc1002.105182. [DOI] [PubMed] [Google Scholar]
- 17.Bolger AP, Coats AJ, Gatzoulis MA. Congenital heart disease: the original heart failure syndrome. Eur Heart J. 2003;24:970–976. DOI: 10.1016/S0195-668X(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 18.Dinardo JA. Heart failure associated with adult congenital heart disease. Semin Cardiothorac Vasc Anesth. 2013;17:44–54. DOI: 10.1177/1089253212469841. [DOI] [PubMed] [Google Scholar]
- 19.Gilljam T, Mandalenakis Z, Dellborg M, Lappas G, Eriksson P, Skoglund K, Rosengren A. Development of heart failure in young patients with congenital heart disease: a nation‐wide cohort study. Open Heart. 2019;6:e000858. DOI: 10.1136/openhrt-2018-000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandalenakis Z, Rosengren A, Lappas G, Eriksson P, Gilljam T, Hansson PO, Skoglund K, Fedchenko M, Dellborg M. Atrial fibrillation burden in young patients with congenital heart disease. Circulation. 2018;137:928–937. DOI: 10.1161/CIRCULATIONAHA.117.029590. [DOI] [PubMed] [Google Scholar]
- 21.Mandalenakis Z, Rosengren A, Lappas G, Eriksson P, Hansson PO, Dellborg M. Ischemic stroke in children and young adults with congenital heart disease. J Am Heart Assoc. 2016;23:e003071. DOI: 10.1161/JAHA.115.003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S, Joseph KS, Lisonkova S, Rouleau J, Van den Hof M, Sauve R, Kramer MS; Canadian Perinatal Surveillance System . Association between maternal chronic conditions and congenital heart defects: a population‐based cohort study. Circulation. 2013;128:583–589. DOI: 10.1161/CIRCULATIONAHA.112.001054. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Joseph KS, Luo W, León JA, Lisonkova S, Van den Hof M, Evans J, Lim K, Little J, Sauve R, et al. Effect of folic acid food fortification in Canada on congenital heart disease subtypes. Circulation. 2016;134:647–655. DOI: 10.1161/CIRCULATIONAHA.116.022126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Botto LD, Lin AE, Riehle‐Colarusso T, Malik S, Correa A; National Birth Defects Prevention Study . Seeking causes: classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Mol Teratol. 2007;79:714–727. DOI: 10.1002/bdra.20403. [DOI] [PubMed] [Google Scholar]
- 25.Zomer AC, Vaartjes I, van der Velde ET, de Jong H, Konings TC, Wagenaar LJ, Heesen WF, Eerens F, Baur L, Grobbee DE, et al. Heart failure admissions in adults with congenital heart disease; risk factors and prognosis. Int J Cardiol. 2013;168:2487–2493. DOI: 10.1016/j.ijcard.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Pandian JD, Sebastian IA, Sidhu A. Acute ischaemic stroke in secondary polycythaemia due to complex congenital cyanotic heart disease. BMJ Case Rep. 2019;12:e231261. DOI: 10.1136/bcr-2019-231261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjork A, Mandalenakis Z, Giang KW, Rosengren A, Eriksson P, Dellborg M. Incidence of Type 1 diabetes mellitus and effect on mortality in young patients with congenital heart defect ‐ a nationwide cohort study. Int J Cardiol. 2020;310:58–63. DOI: 10.1016/j.ijcard.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Fox CK, Sidney S, Fullerton HJ. Community‐based case‐control study of childhood stroke risk associated with congenital heart disease. Stroke. 2015;46:336–340. DOI: 10.1161/STROKEAHA.114.007218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung MG, Guilliams KP, Wilson JL, Beslow LA, Dowling MM, Friedman NR, Hassanein SMA, Ichord R, Jordan LC, Mackay MT, et al. Arterial ischemic stroke secondary to cardiac disease in neonates and children. International Pediatric Stroke Study Investigators. Pediatr Neurol. 2019;100:35–41. DOI: 10.1016/j.pediatrneurol.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. DOI: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fedchenko M, Mandalenakis Z, Hultsberg‐Olsson G, Dellborg H, Eriksson P, Dellborg M. Validation of myocardial infarction diagnosis in patients with congenital heart disease in Sweden. BMC Cardiovasc Disord. 2020;20:460. DOI: 10.1186/s12872-020-01737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S9


