Abstract
Background and Purpose:
Cadmium has been associated with risk of cardiovascular events, including stroke. Human cadmium exposure occurs primarily through diet and tobacco smoke. Recent cohort studies have found an association with stroke, but residual confounding from smoking, could not be ruled out. We therefore conducted a case-cohort study to evaluate whether cadmium is associated with stroke in never-smokers.
Methods:
The Danish Diet Cancer and Health cohort consists of Danes 50-64 years old, recruited in 1993-1997. From never-smoking cohort members without previous cancer or stroke we sampled a sub-cohort of 1200 persons. We also identified all (n=534) cases in the cohort with a validated stroke diagnosis between baseline and 2009. We quantified cadmium and creatinine concentrations from baseline urine samples and used cadmium per creatinine as our main exposure metric. We used Cox proportional hazards models to estimate hazard ratios (HRs) with age as time scale and adjusting for BMI, education and urinary cotinine with and without stratification by sex.
Results:
The median urinary cadmium concentration was 0.21 μg cadmium/g creatinine in cases and 0.19 μg/g in the sub-cohort. The majority (83%) of stroke cases were diagnosed with ischemic stroke. The HR for stroke in the highest quartile of exposure (median 0.44 μg/g creatinine) was 1.11 (95% CI: 0.79-1.54) compared with the lowest quartile (median 0.10 μg/g creatinine). The HR per inter quartile range (IQR, 0.19 μg/g creatinine) was 1.02 (95% CI: 0.92-1.12). Among men, the HR per IQR higher levels of cadmium (0.16 μg/g creatinine) was 1.18 (95% CI: 0.92-1.52), and 1.00 (95% CI: 0.89-1.12) among women. Adjusting for creatinine or using osmolality instead of creatinine standardization generally attenuated observed relationships.
Conclusions:
Our results do not support that low levels of cadmium exposure among never-smokers are strongly associated with risk of stroke, although results varied somewhat by sex and method of accounting for urinary dilution.
Keywords: cadmium, stroke, cardiovascular disease, case-cohort study
Introduction
Stroke is one of the leading causes of morbidity and mortality in the world1. There are numerous established risk factors including hypertension, smoking, sedentary lifestyle, diet, obesity, diabetes, and air pollution2. Increasing evidence suggests that cadmium (Cd) may also pose a risk. Cd is a toxic metal primarily used for battery manufacture3. It is also a contaminant of fertilizers and soil from where it is absorbed by plants3. The primary source of human non-occupational Cd exposure is via diet and smoking4. Studies have found that Cd accumulates in vessel-walls5 and associated Cd with formation of plaques5, atherosclerosis6–8, hypertension9, 10, inflammation11, 12 and a recent study found that in headache patients, levels of Cd in blood was associated with unruptured intracranial aneurysms13. All these factors are associated with risk of stroke2 and epidemiological studies assessing Cd from blood or urine have generally indicated an association between Cd and stroke 7, 14–20. Smoking is a major source of cadmium as well as an established risk factor for stroke2. Levels of urinary cadmium is therefore a marker of smoking and associations observed between Cd and stroke may be due to smoking rather than cadmium specifically . The few generally small, prospective studies attempting to resolve t this issue through sub-analyses restricted to non-smokers have produced conflicting results, ranging from no association to associations found among both smokers and non-smokers 14, 15, 18, 19. Most epidemiological studies on health effects of Cd have focused on all strokes 19 or ischemic strokes 18, 19. A recent study from Sweden found an association between blood Cd and subarachnoid hemorrhages 21, whereas an earlier Swedish study found no association between dietary Cd and hemorrhagic strokes, estimated using a food frequency questionnaire 22, 23. However, dietary questionnaires are not ideally suited to estimate long-term Cd exposure 24, 25. Cd accumulates in the kidneys with a half-life of 10-30 years via urinary excretion, and spot urine measurements provide an effective biomarker of long-term Cd burden26–28.
In the present study, we used urine Cd levels to investigate the relationship between Cd exposure and hazard of stroke in a large population of never smokers.
Methods
Data availability
Data from the Diet, Cancer and Health cohort is not publicly available due to Danish legislation concerning protection of personal data. Admission to accessing data is based on application to the principal investigators Anne Tjønneland and Kim Overvad, through whom the application will be distributed to the steering committee of the Diet, Cancer and Health cohort, who will then process the application and return to the applicant with a final decision regarding access to data. Also, acquisition of data requires compliance with all Danish and EU regulations. The e-mail address for Dr. Anne Tjønneland is: AnneT@Cancer.DK The email address for Dr. Kim Overvad is: KO@PH.AU.DK
Study population
The Danish Diet Cancer and Health (DCH) cohort consists of 57,053 persons aged 50-64 years old at enrollment. The cohort is described in detail elsewhere 29. In brief, participants were recruited during the years 1993-1997 among residents of the two major urban areas in Demark (Aarhus and Copenhagen) and were required to have no diagnosed cancer at enrollment (after recruitment 581 cohort members were excluded due to prevalent cancer not yet registered at baseline). Participants answered an extensive questionnaire and provided a urinary sample at baseline, when a lab technician also took anthropometric measurements. By means of a personal identification number, universally applied in Denmark since 196830, 31, members of the cohort were followed in the extensive nationwide health and administrative registers 32. For the present study, persons were excluded for not providing a urine sample (n=390) or for not having remaining urine in the biobank (n=772). Persons with missing information about smoking status (n=69) were also excluded. Finally, we excluded persons reporting to be current (n=19,916) or former smokers (n=15,931) leaving a study population for the present study of 19,394 persons.
Ethics
The DCH study was approved by the research ethics committee for Copenhagen and Frederiksberg and all participants provided written informed consent at enrollment.
Cases and subcohort
To obtain a representative subcohort, we randomly selected 600 men and 600 women from the DCH cohort. The selection process is illustrated in figure 1. We then identified all potential cases of stroke (ICD10: I60, I61, I63, I64) within the DCH cohort until November 30th, 2009 by linkage to the Danish National Patient register33, 34. A physician with neurological experience reviewed medical records of all potential cases and identified 45 primary strokes in the subcohort and 489 additional primary strokes within the rest of the DCH cohort35. Strokes were defined as cases with rapid onset of focal or global neurological deficit of vascular origin. Either MRI/CT scans should suggest stroke as the cause of the deficit or the deficit should persist beyond 24 hours and lead to death. The identified cases were divided into ischemic and hemorrhagic stroke (further divided into cerebral hemorrhage and subarachnoid hemorrhage) by means of records on CT, MRI, lumbar punctures, and autopsies. Only the first stroke diagnosis for each patient was counted in the present study and only stroke patients diagnosed after baseline were included. Details about the adjudication can be found elsewhere 35.
Figure 1: Flow chart of the case-cohort sampling procedure.
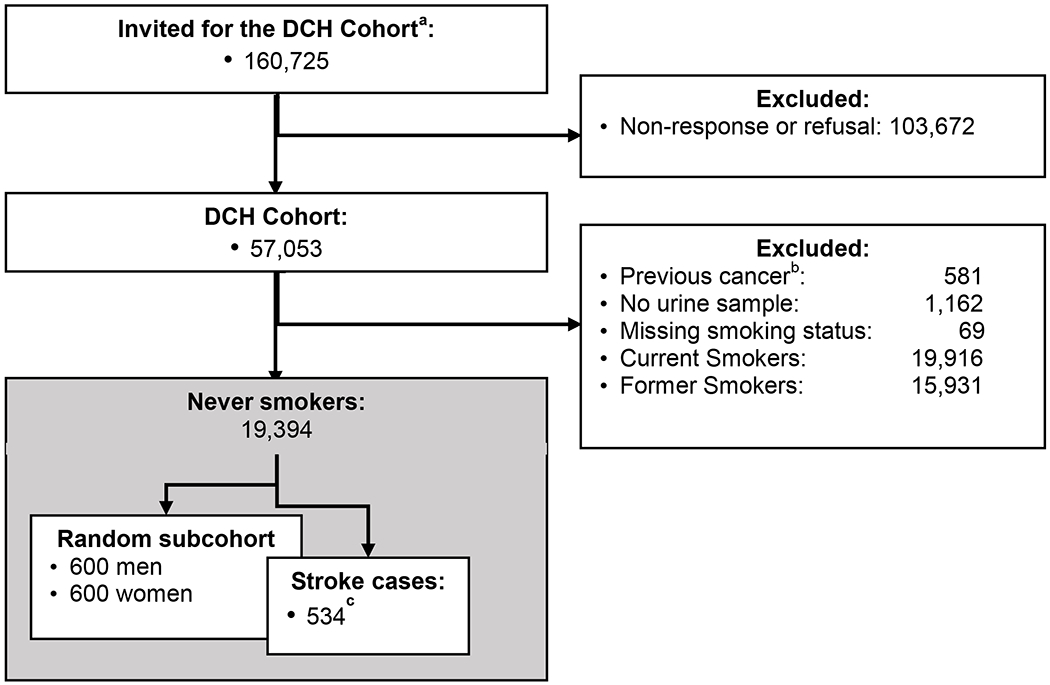
From recruiting the Diet Cancer and Health Cohort (DCH) until the final sample of all never-smoking cases and a random sub cohort.
a: Invitees for the DCH cohort where all cancer free 50-64 year old Danish born residents of Copenhagen and Aarhus area, Denmark; 1993-1997.
b: Cancer diagnosed but not recorded in the Danish Cancer Register prior to recruitment.
c: Of the 534 stroke cases in the never smoking DCH members, 45 were also part of the randomly sampled subcohort.
Cadmium and other urinary elements
For all members of the DCH cohort, spot urine samples were collected at baseline using trace-metal free methodology and the sample was deposited in a biobank. For each member of our subcohort and for all cases, a 1 ml sample was analyzed at RTI International’s Trace Metals Laboratory (Research Triangle Park, NC). Urinary samples from cases and subcohort members were analyzed in batches mixed and anonymized before shipment to the laboratory to ensure completely blinded analysis and to account for potential batch-to-batch variation in urine analysis. Details about the analysis of urinary samples as well as quality control can be found in the Data Supplement. In brief, we used an iCAP Q ICP-MS system (Thermo Scientific, Waltham, MA) equipped with a collision cell to determinate a suite of 19 elements including cadmium. The instrument was tuned daily to optimize sensitivity across the mass range and mitigate polyatomic interferences through use of the collision cell. Multiple isotopes were monitored for most elements to further account for potential analytical interferences, such as molybdenum for cadmium.
Urinary cotinine was measured by a cotinine ELISA bioassay kit by Abnova Corporation (Taipei, Taiwan). Urinary creatinine was quantified colorimetrically by the Jaffe reaction with a Cayman Chemicals (Ann Arbor, MI) Creatinine Assay Kit. Urinary osmolality was measured by a Model 3320 Micro-Osmometer by Advanced Instruments, Inc. (Norwood, MA).
Covariates
For each participant, we obtained information about potential confounders identified a priori. Apart from age and sex, information about hypertension, highest attained educational level, secondhand smoke exposure at work or at home, and whether participants participated in sports was self-reported at baseline. For women, we also obtained information about parity and menopausal status. At baseline, BMI was calculated for all participants. Finally, we obtained information about first diagnosis of diabetes from the Danish diabetes register, which holds records on all cases of diabetes in Denmark since 199536.
Statistical analysis
In an unweighted or “Prentice” case-cohort approach, we used Cox proportional hazards models, to estimate hazard ratios (HRs) for stroke37 (We tested correlations of event time and scaled Schoenfeld residuals using tests adapted to the case-cohort setting,38 we did not find sufficient evidence to support deviations from proportional hazards: p-values ≥0.12). We used age as the timescale. Subcohort members were followed from baseline (i.e.: date of interview) until Nov 30, 2009, death (n=64), or stroke diagnosis, whichever came first (median follow-up time (25th, 75th percentile) for subcohort members: 13.5 (12.9, 14.2) years).
We calculated HRs for the three upper exposure quartiles with the first quartile as the reference, and tested for linear trend in this quartiles analysis assigning observations in each quartile of creatinine-standardized cadmium the median concentration of the quartile.
We also calculated HRs per IQR difference in creatinine-standardized Cd concentration. To evaluate linearity in this analysis we visually inspected natural cubic spline (knots at 1st and 2nd tertile) and we used likelihood ratio tests to compare this spline model to a linear model interpreting the p-value as the p-value for non-linearity39. The IQR and cut-off points for quartiles were calculated among subcohort members. In the primary analysis, we calculated the HRs associated with Cd standardized by creatinine concentration (μg/g creatinine) in three models: 1) Adjusting for sex and age (time scale), 2) additionally adjusting for cotinine (continuous-linear term) and 3) (our primary fully adjusted model) with additional adjustment for BMI (continuous-linear term) and education (<8yrs, 8-10yrs and >10yrs). We also tested for effect modification by sex and conducted separate analysis for men and women with additional adjustment for menopausal status for the latter. Finally, we analyzed ischemic and hemorrhagic stroke separately censoring subjects if they had the other sub-type of stroke because we only had information about the first stroke.
In sensitivity analyses, we estimated the impact of substituting creatinine standardized cadmium concentrations below level of detection (LOD) (Median (25th, 75th) Cd LOD across batches= 0.002 (0.0008, 0.003); N< LOD= 28) with LOD/√2. We also conducted analyses restricted to the 5-95th percentile of creatinine standardized cadmium concentration to avoid potential undue impact of extreme observations.
In further sensitivity analyses, we investigated if the results were robust to different ways of accounting for urinary dilution: 1) we adjusted for creatinine; 2) we performed covariate-adjusted standardization as suggested by O’Brien et al (2016)40; 3) we adjusted for osmolality instead of for creatinine, and 4) we standardized the urinary concentration for osmolality, these analyses were also done stratified by sex. Some studies have suggested an association between Cd and diabetes which is an established risk factor for stroke. However, diabetes and kidney function are also potential intermediaries on the pathway between Cd and stroke. We therefore performed sensitivity analyses, either excluding all participants with diabetes or excluding prevalent diabetes cases at baseline and adjusting for diabetes diagnosed during follow-up. We also did sensitivity analyses: 1) Adjusting for diabetes, hypertension and hypocholesteremia 2) Excluding all participants with urinary cotinine ≥200ng/ml as these might be current smokers41. 3) Excluding participants with extreme creatinine levels (<0.03 g/L or >3g/L).
All analyses were conducted in R version 3.6.2 using the survival package and functions to account for the case-cohort study design.
Results:
Compared to subcohort members, cases were slightly older, more likely to be female and to have less education (Table 1). Cases had higher BMI, and female cases were less likely to be premenopausal. Cases were more likely than subcohort members to report exposure to second hand smoke after age 50 and had slightly higher urinary cadmium levels. In the subcohort, high levels of creatinine standardized urinary cadmium were associated with older age and being female (Table 1 in the data supplement). When comparing to the 1st quartile of creatine standardized cadmium levels the HRs for 3rd and 4th quartile were 1.10 (95% CI: 0.81-1.59) and 1.11 (95% CI: 0.79-1.55) respectively (p-value for trend=0.56) (Table 2). The fully adjusted HR for stroke per 0.19 μg/g difference in cadmium levels was 1.02 (95% CI: 0.93-1.13). The spline plot provided some indication of elevated HR at the highest levels of exposure (Figure 2). All HR estimates, however, had wide confidence intervals reaching well above and below the null. HR estimates from the simpler models 1 and 2 were similar to the fully adjusted estimates.
Table 1.
Baseline characteristics of stroke cases and subcohort members.
| Baseline characteristics | Subcohort (n=1200) | Stroke cases (n=534) | ||
|---|---|---|---|---|
| % (No) | Median (25th, 75th) | % (No) | Median (25th, 75th) | |
| Age at enrollment (years) | 100 (1200) | 55.8 (52.5, 59.9) | 100 (534) | 58.4 (54.2, 62.0) |
| Sex | ||||
| Male | 50.0 (600) | 48.1 (257) | ||
| Female | 50.0 (600) | 51.9 (277) | ||
| Education | ||||
| Low (<8 years) | 28.7 (344) | 35.0 (187) | ||
| Medium (8-10 years) | 45.8 (549) | 41.6 (222) | ||
| High (>10 years) | 25.6 (307) | 23.4 (125) | ||
| Parity (women only) | ||||
| 0 | 12.8 (77) | 14.0 (36) | ||
| 1 to 2 | 60.0 (360) | 58.4 (150) | ||
| 3 to 8 | 27.2 (163) | 27.6 (71) | ||
| Menopausal status (women only) | ||||
| Post | 58.2 (349) | 63.8 (164) | ||
| Pre | 17.2 (103) | 9.7 (25) | ||
| Unknown | 24.7 (148) | 26.5 (68) | ||
| Cotinine concentration | ||||
| ≤ 20 ng/mL | 57.5 (690) | 51.1 (273) | ||
| >20 ng/ml - <50 ng/mL | 29.5 (354) | 34.6 (185) | ||
| ≥50 ng/mL - <200ng/mL | 11.2 (134) | 11.4 (61) | ||
| ≥200 ng/mL | 1.8 (22) | 2.8 (15) | ||
| Self-reported secondhand smoke exposure at home or work | ||||
| None | 2.2 (26) | 2.1 (11) | ||
| Only before age 50 | 27.8 (334) | 22.3 (119) | ||
| After age 50 | 70.0 (840) | 75.7 (404) | ||
| BMI | ||||
| < 25 | 39.9 (479) | 31.1 (166) | ||
| 25 to < 30 | 43.6 (523) | 45.9 (245) | ||
| 30 + | 16.5 (198) | 23.0 (123) | ||
| Urine Cd concentration (ng/mL) | 0.19 (0.09, 0.34) | 0.21 (0.10, 0.35) | ||
Table 2.
Stroke risk per interquartile range (IQR = 0.19) difference and quartile of urinary cadmium concentration (creatinine standardized).
| Cd Variable | Total | Cases (n) | Urinary Cd Median μg/g | Model 1 a HR (95% CI) | Model 2 b HR (95% CI) | Model 3 c HR (95% CI) | Trend Test P-value |
|---|---|---|---|---|---|---|---|
| Continuous | 1689 | 534 | 0.204 | 1.02 (0.92-1.12) | 1.02 (0.92-1.12) | 1.02 (0.93-1.13) | 0.56 |
| Quartile 1 | 403 | 119 | 0.098 | Ref | ref | Ref | |
| Quartile 2 | 423 | 132 | 0.166 | 1.05 (0.77-1.41) | 1.03 (0.76-1.39) | 1.03 (0.76-1.40) | |
| Quartile 3 | 422 | 136 | 0.250 | 1.10 (0.81-1.50) | 1.10 (0.81-1.49) | 1.10 (0.81-1.50) | |
| Quartile 4 | 441 | 147 | 0.436 | 1.11 (0.79-1.54) | 1.09 (0.78-1.52) | 1.11 (0.79-1.55) |
Model with age as time axis and adjusted for gender.
Model 1 with additional adjustment for cotinine (continuous).
Fully adjusted model: Model 2 with additional adjustment for BMI (continuous) and education (categorical).
Figure 2.
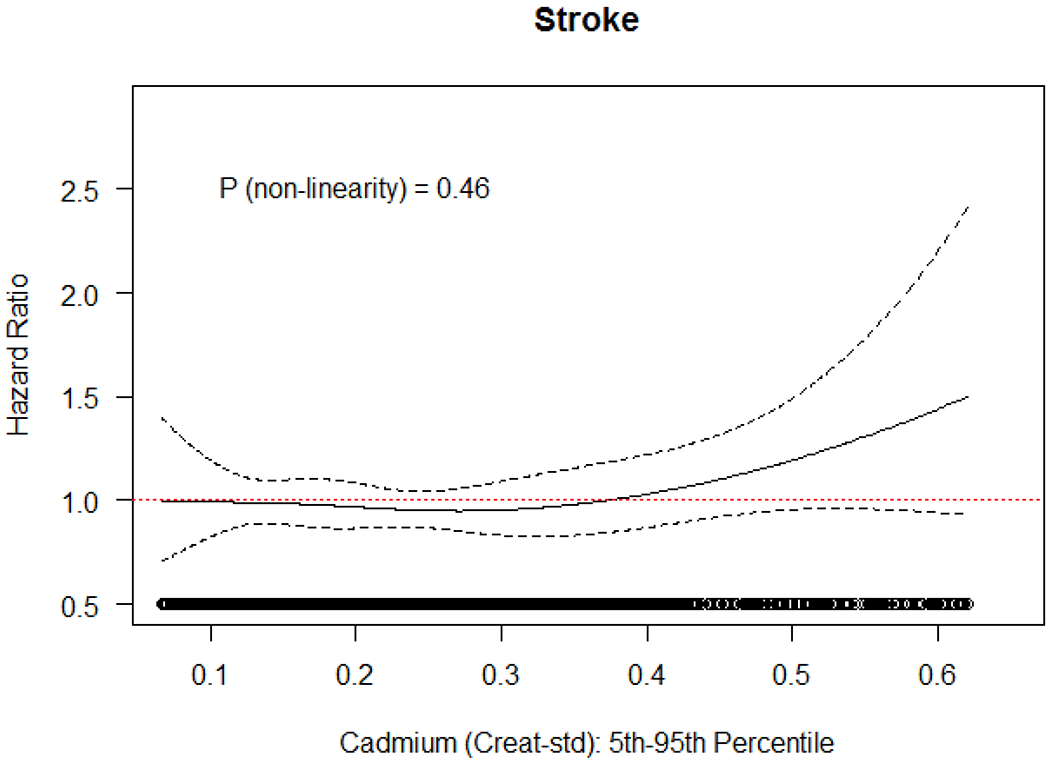
Association and 95% confidence interval between Cd (creatinine standardized) and risk of stroke, with adjustment for age (underlying time axis), sex, BMI (continuous), education (categorical), and cotinine (continuous). Natural cubic splines with three degrees of freedom, restricted to subjects with Cd concentration between 5th and 95th percentile of the exposure distribution.
The p-value for effect modification by sex was 0.01. In analysis stratified by sex, the HR for women was close to the null. For men the HR per IQR was 1.18 (95% CI: 0.92-1.52) and when comparing the highest quartile of exposure (median=0.44 μg/g creatinine) to the lowest quartile (median=0.01 μg/g creatinine) the HR was 1.56 (0.89-2.73) with suggestion of dose response across intermediary quartiles (Table 3). Spline plots were also more indicative of a positive association in males (Figure 1 in the Data Supplement). However, for both men and women most HRs had wide confidence intervals.
Table 3.
Modification of the association between stroke and urinary cadmium concentration (creatinine standardized) by sex.
| U-Cd | Cases n | Urinary Cd median | Adjusted HR a (95% CI) | Cases n | Urinary Cd Median | Adjusted HR a (95% CI) |
|---|---|---|---|---|---|---|
| Sex | Male | Female | ||||
| Continuous* | 277 | 0.160 | 1.18 (0.92-1.52) | 257 | 0.290 | 1.00 (0.89-1.12) |
| Quartile 1 | 92 | 0.100 | Ref | 27 | 0.094 | Ref |
| Quartile 2 | 87 | 0.166 | 1.06 (0.73-1.54) | 45 | 0.167 | 1.08 (0.61-1.93) |
| Quartile 3 | 69 | 0.245 | 1.25 (0.84-1.87) | 67 | 0.256 | 1.03 (0.60-1.77) |
| Quartile 4 | 29 | 0.398 | 1.56 (0.89-2.73) | 118 | 0.454 | 0.98 (0.59-1.64) |
Per IQR (0.19 μg/g) according to the distribution among the 1200 subcohort members
Fully adjusted model with age as time axis and adjusted for BMI (continuous), education (categorical), and cotinine (continuous). For women additionally adjusted for menopausal status
The majority of stroke cases were ischemic (cases=445) and results for this group were similar to the overall analysis (Table 4, Figure 3). There were only 89 cases of hemorrhagic stroke (63 cerebral and 26 subarachnoid hemorrhages), the HR per IQR for these was 1.04 (95% CI: 0.86-1.26) and categorical analysis and spline plots were generally closer to the null.
Table 4.
Association between types of stroke and urinary cadmium concentration (creatinine standardized).
| U-Cd | Cases n | Urinary Cd Median | Adjusted HR (95% CI)a | Urinary Cd Median | Cases n | Adjusted HR (95% CI) a |
|---|---|---|---|---|---|---|
| Ischemic stroke | Hemorrhagic stroke | |||||
| Continuous* | 445 | 1.02 (0.92-1.14) | 0.290 | 89 | 1.04 (0.86-1.26) | |
| Quartile 1 | 101 | 0.098 | Ref | 0.094 | 17 | Ref |
| Quartile 2 | 104 | 0.166 | 0.95 (0.69-1.33) | 0.167 | 28 | 1.55 (0.82-2.91) |
| Quartile 3 | 116 | 0.250 | 1.12 (0.80-1.56) | 0.256 | 20 | 1.07 (0.54-2.13) |
| Quartile 4 | 123 | 0.436 | 1.12 (0.78-1.62) | 0.454 | 24 | 1.09 (0.53-2.24) |
Per IQR (0.19, calculated among all 1200 subcohort members)
Fully adjusted model with age as time axis and adjusted for gender, BMI (continuous), education (categorical), and cotinine (continuous).
Figure 3.
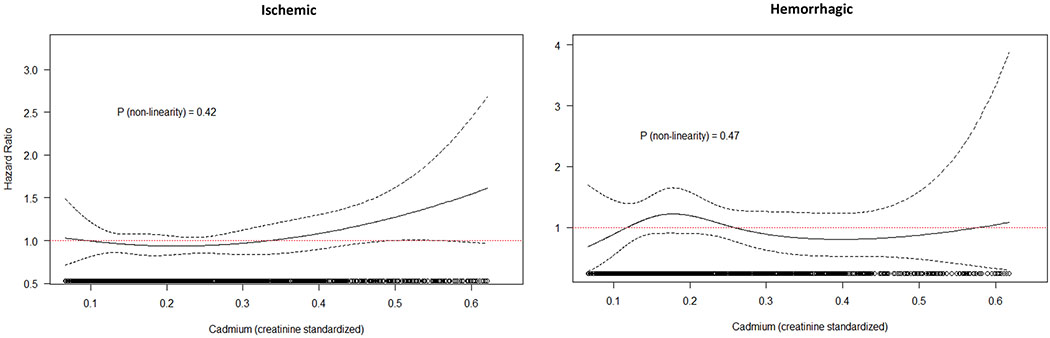
Association and 95% confidence interval between Cd (creatinine standardized) and risk of ischemic and hemorrhagic stroke, with adjustment for age (underlying time axis), sex, BMI (continuous), education (categorical), and cotinine (continuous). Natural cubic splines with three degrees of freedom, restricted to subjects with Cd concentration between 5th and 95th percentile of the exposure distribution
In sensitivity analysis setting creatinine standardized cadmium values below the limit of detection to LOD/√2 42 or excluding extreme values (outside 5-95th percentile) did not substantially change HR estimates (Table 2 in the Data Supplement). Alternative ways of accounting for urinary dilution produced HR per IQR ranging from 1.00 (95% CI: 0.89-1.13) when adjusting for creatinine to 1.06 (95% CI: 0.97-1.17) when standardizing for osmolality (Table 3 and 4 in the Data Supplement). The categorical estimates when using alternative ways to account for urinary dilution were quite variable and there was little evidence of positive dose response. The HRs associated with highest quartile of exposure ranged from 0.92 (95% CI: 0.60-1.41) when adjusting for creatinine to 1.08 (95% CI: 0.65-1.47) when using covariate adjusted creatinine standardization (Table 3 and 4 in the Data Supplement). In these sensitivity analyses, all confidence intervals were wide enough to be compatible with both our main model and the null.
Sex stratified analysis standardizing for osmolality produced HRs of 1.03 (0.87-1.20) in men and 1.09 (0.97-1.21) in women (Table 5 in the Data Supplement).
Finally, we saw similar results when excluding all diabetics or excluding diabetics and adjusting for later incident diabetes, or adjusting for diabetes, hypertension and hypocholesteremia, or when excluding participants with high levels of urinary cotinine or with creatinine levels outside 0.03-3 g/L. (Table 6 and 7 in the Data Supplement).
Discussion
In this large prospective study of never smokers with up to 16 years of follow-up, we found little evidence that at low levels of urine Cd higher exposure levels might be associated with a higher hazard of stroke. There were weak indications of a moderately higher hazard of stroke among males. The evidence of association was attenuated when accounting for urinary dilution by other means than creatinine standardization. Due to small number of hemorrhagic cases we could not determine with confidence whether findings differed by stroke subtype.
Several prior studies have reported positive associations between blood or urinary Cd and risk of stroke7, 14–16, 18–20. Many of these studies have adjusted for smoking, but could not conclude if the observed associations related to Cd exposure independently of smoking7, 16, 20. Few studies have presented results specifically for never smokers14, 15, 18, 19, 21.
In a large Chinese case-control study with 1,016 never-smoking cases, Cd concentrations measured in plasma (which are not usually used in epidemiological studies, but were correlated with concentrations in whole blood) after diagnosis were associated with a fourfold (OR 4.08 95% CI: 2.75-6.06, 152 cases) higher risk of ischemic stroke comparing the fourth and first quartile14. In the Swedish prospective Malmö Diet and Cancer cohort with 111 strokes identified among never-smokers, the HRs for all stroke and ischemic stroke were 2.2 (95% CI: 1.0-4.8) and 2.5 (95% CI: 1.0-6.0), respectively, when comparing the upper to the lower quartile of blood Cd concentration19. In this Swedish cohort study, Cd levels were comparable to the ones observed in the present study, there were, however, only seven stroke cases at the highest exposure level, and there was no evidence of elevated risk at lower levels of exposure. Two studies in the United States have analyzed urinary Cd in never-smokers. Among Native Americans in the prospective Strong Heart Study with 68 never-smoking stroke cases with high levels of Cd, there was not strong evidence of an association between Cd and stroke (HR=0.90 (95% CI: 0.49-1.65) when comparing the 80th (1.52 μg/g creatinine) and 20th (0.55 μg/g creatinine) percentile of creatinine corrected Cd concentration15, respectively. In the prospective REGARDS case-cohort study with 288 never-smoking cases, creatinine corrected Cd levels in the third tertile (>0.56μg/g creatinine) were compared to the first tertile (<=0.31μg/g creatinine) and yielded a HR for stroke of 1.27 (95% CI: 0.80-2.03) 18. The wide confidence intervals in these studies span the null compatible with the lack of strong association suggested by the present study. Even if some bias towards the null occurred due to exposure misclassification, the age of participants and the assessment of Cd from urine minimizes the likely impact on our results as discussed under strengths and limitations. It should, however, be noted that our categorical results are statistically compatible with higher risk as suggested by previous studies, illustrating that the limited precision of estimates and differences in methodology to some extent hamper direct comparison of studies.
A recent study also using the Malmö Diet and Cancer Cohort reported blood cadmium to be associated with a higher risk of subarachnoid hemorrhage 21. This association appeared largely attributable to smoking. Among never and former smokers the observed OR was 1.40 (95% CI: 0.27-7.16) when comparing fourth quartile to the 1st-3rd quartile combined. Due to lack of precision, neither this result nor the ones from the present study, allow any conclusion regarding whether Cd is a risk factor for hemorrhagic stroke in never-smokers.
In women, creatinine-corrected levels of urinary Cd are generally higher than in men, which may be attributable to lower levels of creatinine and iron. In a review of the literature on Cd and cardiovascular disease, all individual studies reported positive associations in men whereas results were mixed for women but there was no apparent difference between sexes in a meta-analysis16. In our data, indications of a potential association with creatinine standardized Cd were weak and only apparent in males, and we cannot exclude the possibility that this resulted from chance. We are not aware of other studies that have investigated sex differences in the association between Cd and stroke among never smokers. When including smokers, the Chinese study14 and the American REGARDS18 study did not find differences by sex whereas the Strong Heart Study found stronger associations in men15 and the Swedish Diet and Health Cohort found stronger associations in women19.
In our main model, we controlled for variation in urine density by creatinine standardization as this a widely used approach that has shown high within person stability in samples collected years apart 28, 43. There is however, no established consensus on how to best account for urinary dilution and we therefore also investigated other methods. In general these additional approaches produced even weaker indications of an association than creatinine standardization, especially in the categorical analyses. We have no good explanation for this but cautious interpretation of our main analyses is merited.
Strengths and limitations
It was a major strength of our study that that we could prospectively follow a large population consisting exclusively of never-smokers, over many years, in a country with high quality registers and free universal health care31, 32. This allowed for almost complete follow-up of the cohort as well as identification of incident cases from independent registers with little risk of socioeconomic position influencing likelihood of being registered as a case. Hospital discharge diagnoses of stroke, particularly for outpatients, have been found to have limited validity. It was therefore a strength that all stroke cases were adjudicated by experts 35, which was done independently of the present study and thus blinded to exposure status. However, this limited our sample of stroke cases to those diagnosed prior to 2010. We also used completely blinded state-of-the-art methodology to quantify Cd in urine, which is considered a near ideal proxy for long-term Cd load44.
Furthermore, we accounted for urinary dilution in multiple ways using either creatinine or osmolality alerting us to the potential impact of how this was addressed. It was also a strength that urine samples were collected before diagnosis and while we only had one urinary spot sample for each participant several studies have demonstrated high intraclass correlation between Cd levels in samples taken weeks, months or years apart 28, 43, 45. We adjusted for urinary cotinine rather than self-reported passive smoking, and even though both metrics address exposure around baseline, use of cotinine captures both second- and third-hand smoke exposure, is highly sensitive, and is not susceptible to reporting bias46. Our study also had some potential limitations. Thirtyfive percent of those invited were enrolled into the DCH-cohort. Participants were better educated, had higher incomes and were more likely to be married29. We do, however, not expect the biological effect of Cd to differ between participants and non-participants. In analysis of ischemic and hemorrhagic stroke we had to censor participants when they had the other type of stroke. The other stroke type can thus be perceived as a competing risk. The censoring, however, addresses that cases are at an increased risk of having another stroke47 and that they may change lifestyle and receive medication after a diagnosis of stroke.
Our prospective study has more never-smoking cases than any study published to date, however, the precision of our estimates was still limited. It is a potential limitation that all questionnaire data were only available at baseline. However, the relatively high age of participants at baseline limits the likelihood that participants took up smoking when they had never smoked before48. Similarly the majority are unlikely to have changed their dietary habits substantially.
The exposure levels in the present study are comparable to or slightly lower than the exposure levels observed in non-smokers in the USA49 and our study population was almost exclusively Caucasian. Thus, our results can probably be generalized to Caucasian western populations.
Conclusion
This large prospective cohort with up to 16 years of follow-up provides little evidence that low levels of cadmium may be a risk factor for stroke; although a hint of an association was observed among never-smoking men. Further studies are needed as all confidence intervals were wide and results were sensitive to how urinary dilution was addressed.
Supplementary Material
Acknowledgments
Sources of Funding
Funding for this work came from NIEHS R01ES025514. The establishment and running of The Diet, Cancer and Health cohort was funded by the Danish Cancer Society. The funding sources had no part in the collection, analysis or interpretation of data and they did not participate in the writing of the report or in the decision to submit the article for publication
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclosures
Dr. Wellenius has received consulting fees from the Health Effects institute (Boston, MA) and serves as a paid visiting scientist at Google Research. Other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplement Materials
• Analysis of urinary elements: Detailed methods
References
- 1.Collaborators GBDS. Global, regional, and national burden of stroke, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18:439–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120:472–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan MA, Khan S, Khan A, Alam M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci Total Environ. 2017;601-602:1591–1605 [DOI] [PubMed] [Google Scholar]
- 4.Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, Ingerman L, et al. Toxicological profile for cadmium. Toxicological profile for cadmium. Atlanta (GA); 2012. [PubMed] [Google Scholar]
- 5.Bergstrom G, Fagerberg B, Sallsten G, Lundh T, Barregard L. Is cadmium exposure associated with the burden, vulnerability and rupture of human atherosclerotic plaques? PloS one. 2015;10:e0121240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira TF, Batista PR, Leal MA, Campagnaro BP, Nogueira BV, Vassallo DV, et al. Chronic cadmium exposure accelerates the development of atherosclerosis and induces vascular dysfunction in the aorta of apoe(−/−) mice. Biol Trace Elem Res. 2019;187:163–171 [DOI] [PubMed] [Google Scholar]
- 7.Tinkov AA, Filippini T, Ajsuvakova OP, Skalnaya MG, Aaseth J, Bjorklund G, et al. Cadmium and atherosclerosis: A review of toxicological mechanisms and a meta-analysis of epidemiologic studies. Environmental research. 2018;162:240–260 [DOI] [PubMed] [Google Scholar]
- 8.Fagerberg B, Barregard L, Sallsten G, Forsgard N, Ostling G, Persson M, et al. Cadmium exposure and atherosclerotic carotid plaques--results from the malmo diet and cancer study. Environmental research. 2015;136:67–74 [DOI] [PubMed] [Google Scholar]
- 9.Oliver-Williams C, Howard AG, Navas-Acien A, Howard BV, Tellez-Plaza M, Franceschini N. Cadmium body burden, hypertension, and changes in blood pressure over time: Results from a prospective cohort study in american indians. J Am Soc Hypertens. 2018;12:426–437 e429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Guallar E. Cadmium exposure and hypertension in the 1999-2004 national health and nutrition examination survey (nhanes). Environmental health perspectives. 2008;116:51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olszowski T, Baranowska-Bosiacka I, Gutowska I, Chlubek D. Pro-inflammatory properties of cadmium. Acta Biochim Pol. 2012;59:475–482 [PubMed] [Google Scholar]
- 12.Fagerberg B, Borne Y, Barregard L, Sallsten G, Forsgard N, Hedblad B, et al. Cadmium exposure is associated with soluble urokinase plasminogen activator receptor, a circulating marker of inflammation and future cardiovascular disease. Environmental research. 2017;152:185–191 [DOI] [PubMed] [Google Scholar]
- 13.Yoon BNR, Lee JB, Jin GH, Kim WY. Serum cadmium level is positively associated with unruptured intracranial aneurysm incidence. Korean J Fam Med. 2019;40:273–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen Y, Huang S, Zhang Y, Zhang H, Zhou L, Li D, et al. Associations of multiple plasma metals with the risk of ischemic stroke: A case-control study. Environment international. 2019;125:125–134 [DOI] [PubMed] [Google Scholar]
- 15.Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, et al. Cadmium exposure and incident cardiovascular disease. Epidemiology. 2013;24:421–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tellez-Plaza M, Jones MR, Dominguez-Lucas A, Guallar E, Navas-Acien A. Cadmium exposure and clinical cardiovascular disease: A systematic review. Curr Atheroscler Rep. 2013;15:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tellez-Plaza M, Navas-Acien A, Menke A, Crainiceanu CM, Pastor-Barriuso R, Guallar E. Cadmium exposure and all-cause and cardiovascular mortality in the u.S. General population. Environmental health perspectives. 2012;120:1017–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Xun P, Tsinovoi C, McClure LA, Brockman J, MacDonald L, et al. Urinary cadmium concentration and the risk of ischemic stroke. Neurology. 2018;91:e382–e391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barregard L, Sallsten G, Fagerberg B, Borne Y, Persson M, Hedblad B, et al. Blood cadmium levels and incident cardiovascular events during follow-up in a population-based cohort of swedish adults: The malmo diet and cancer study. Environmental health perspectives. 2016;124:594–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borne Y, Fagerberg B, Persson M, Ostling G, Soderholm M, Hedblad B, et al. Cadmium, carotid atherosclerosis, and incidence of ischemic stroke. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soderholm M, Borne Y, Hedblad B, Persson M, Barregard L, Engstrom G. Blood cadmium concentration and risk of subarachnoid haemorrhage. Environmental research. 2020;180:108826. [DOI] [PubMed] [Google Scholar]
- 22.Julin B, Wolk A, Thomas LD, Akesson A. Exposure to cadmium from food and risk of cardiovascular disease in men: A population-based prospective cohort study. Eur J Epidemiol. 2013;28:837–840 [DOI] [PubMed] [Google Scholar]
- 23.Julin B, Bergkvist C, Wolk A, Akesson A. Cadmium in diet and risk of cardiovascular disease in women. Epidemiology. 2013;24:880–885 [DOI] [PubMed] [Google Scholar]
- 24.Vacchi-Suzzi C, Eriksen KT, Levine K, McElroy J, Tjonneland A, Raaschou-Nielsen O, et al. Dietary intake estimates and urinary cadmium levels in danish postmenopausal women. PloS one. 2015;10:e0138784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quraishi SM, Adams SV, Shafer M, Meliker JR, Li W, Luo J, et al. Urinary cadmium and estimated dietary cadmium in the women’s health initiative. J Expo Sci Environ Epidemiol. 2016;26:303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amzal B, Julin B, Vahter M, Wolk A, Johanson G, Akesson A. Population toxicokinetic modeling of cadmium for health risk assessment. Environmental health perspectives. 2009;117:1293–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akerstrom M, Barregard L, Lundh T, Sallsten G. The relationship between cadmium in kidney and cadmium in urine and blood in an environmentally exposed population. Toxicol Appl Pharmacol. 2013;268:286–293 [DOI] [PubMed] [Google Scholar]
- 28.Vacchi-Suzzi C, Kruse D, Harrington J, Levine K, Meliker JR. Is urinary cadmium a biomarker of long-term exposure in humans? A review. Curr Environ Health Rep. 2016;3:450–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tjonneland A, Olsen A, Boll K, Stripp C, Christensen J, Engholm G, et al. Study design, exposure variables, and socioeconomic determinants of participation in diet, cancer and health: A population-based prospective cohort study of 57,053 men and women in denmark. Scandinavian journal of public health. 2007;35:432–441 [DOI] [PubMed] [Google Scholar]
- 30.Schmidt M, Pedersen L, Sorensen HT. The danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549 [DOI] [PubMed] [Google Scholar]
- 31.Pedersen CB. The danish civil registration system. Scand. J Public Health. 2011;39:22–25 [DOI] [PubMed] [Google Scholar]
- 32.Thygesen LC, Daasnes C, Thaulow I, Bronnum-Hansen H. Introduction to danish (nationwide) registers on health and social issues: Structure, access, legislation, and archiving. Scand. J Public Health. 2011;39:12–16 [DOI] [PubMed] [Google Scholar]
- 33.Lynge E, Sandegaard JL, Rebolj M. The danish national patient register. Scand. J Public Health. 2011;39:30–33 [DOI] [PubMed] [Google Scholar]
- 34.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The danish national patient registry: A review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luhdorf P, Overvad K, Schmidt EB, Johnsen SP, Bach FW. Predictive value of stroke discharge diagnoses in the danish national patient register. Scandinavian journal of public health. 2017;45:630–636 [DOI] [PubMed] [Google Scholar]
- 36.Carstensen B, Kristensen JK, Marcussen MM, Borch-Johnsen K. The national diabetes register. Scand. J Public Health. 2011;39:58–61 [DOI] [PubMed] [Google Scholar]
- 37.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. Journal of clinical epidemiology. 1999;52:1165–1172 [DOI] [PubMed] [Google Scholar]
- 38.Xue X, Xie X, Gunter M, Rohan TE, Wassertheil-Smoller S, Ho GY, et al. Testing the proportional hazards assumption in case-cohort analysis. BMC Med Res Methodol. 2013;13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodward M Epidemiology: Study design and data analysis, second edition. United States of America: Chapman & Hall/CRC; 2009. [Google Scholar]
- 40.O’Brien KM, Upson K, Cook NR, Weinberg CR. Environmental chemicals in urine and blood: Improving methods for creatinine and lipid adjustment. Environmental health perspectives. 2016;124:220–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim S Overview of cotinine cutoff values for smoking status classification. International journal of environmental research and public health. 2016;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hornung RWR LD Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene. 1990;5:46–51 [Google Scholar]
- 43.Meliker JR, Vacchi-Suzzi C, Harrington J, Levine K, Lui LY, Bauer DC, et al. Temporal stability of urinary cadmium in samples collected several years apart in a population of older persons. International journal of hygiene and environmental health. 2019;222:230–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238:201–208 [DOI] [PubMed] [Google Scholar]
- 45.Vacchi-Suzzi C, Porucznik CA, Cox KJ, Zhao Y, Ahn H, Harrington JM, et al. Temporal variability of urinary cadmium in spot urine samples and first morning voids. J Expo Sci Environ Epidemiol. 2017;27:306–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torres S, Merino C, Paton B, Correig X, Ramirez N. Biomarkers of exposure to secondhand and thirdhand tobacco smoke: Recent advances and future perspectives. International journal of environmental research and public health. 2018;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohan KM, Wolfe CD, Rudd AG, Heuschmann PU, Kolominsky-Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: A systematic review and meta-analysis. Stroke. 2011;42:1489–1494 [DOI] [PubMed] [Google Scholar]
- 48.Weinberger AH, Pilver CE, Mazure CM, McKee SA. Stability of smoking status in the us population: A longitudinal investigation. Addiction. 2014;109:1541–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for disease control and prevention. Fourth report on human exposure to environmental chemicals, updated tables, (january 2019).2020 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the Diet, Cancer and Health cohort is not publicly available due to Danish legislation concerning protection of personal data. Admission to accessing data is based on application to the principal investigators Anne Tjønneland and Kim Overvad, through whom the application will be distributed to the steering committee of the Diet, Cancer and Health cohort, who will then process the application and return to the applicant with a final decision regarding access to data. Also, acquisition of data requires compliance with all Danish and EU regulations. The e-mail address for Dr. Anne Tjønneland is: AnneT@Cancer.DK The email address for Dr. Kim Overvad is: KO@PH.AU.DK


