Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific antibodies, particularly those preventing interaction between the viral spike receptor-binding domain and the host angiotensin-converting enzyme 2 receptor, may prevent viral entry into host cells and disease progression.
Methods
We performed a systematic review, meta-analysis, trial sequential analysis (TSA), and meta-regression of RCTs to evaluate the benefit of convalescent plasma for COVID-19. The primary outcome was 28–30 day mortality. Secondary outcomes included need for mechanical ventilation and ICU admission. Data sources were PubMed, Embase, MedRxiv, and the Cochrane library on July 2, 2021.
Results
We identified 17 RCTs that recruited 15 587 patients with 8027 (51.5%) allocated to receive convalescent plasma. Convalescent plasma use was not associated with a mortality benefit (24.7% vs 25.5%; odds ratio [OR]=0.94 [0.85–1.04]; P=0.23; I2=4%; TSA adjusted confidence interval [CI], 0.84–1.05), or reduction in need for mechanical ventilation (15.7% vs 15.4%; OR=1.01 [0.92–1.11]; P=0.82; I2=0%; TSA adjusted CI, 0.91–1.13), or ICU admission (22.4% vs 16.7%; OR=0.80 [0.21–3.09]; P=0.75; I2=63%; TSA adjusted CI, 0.0–196.05). Meta-regression did not reveal association with titre of convalescent plasma, timing of administration, or risk of death and treatment effect (P>0.05). Risk of bias was high in most studies.
Conclusions
In patients with COVID-19, there was no clear mortality benefit associated with convalescent plasma treatment. In patients with mild disease, convalescent plasma did not prevent either the need for mechanical ventilation or ICU admission.
Clinical trial registration
CRD42021234201 (PROSPERO).
Keywords: antibodies, convalescent plasma, COVID-19, meta-analysis, passive immunisation
Editor's key points.
-
•
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific antibodies can neutralise the virus. The benefit of convalescent plasma in the management of patients with COVID-19 requires evaluation.
-
•
In this systematic review, the authors reviewed 17 RCTs including 15 587 subjects. There was no clear mortality benefit associated with the use of convalescent plasma, nor any reduction in the need for mechanical ventilation or ICU admission.
-
•
There appears to be no benefit associated with convalescent plasma in the management of patients with COVID-19.
-
•
The benefit of high-titre convalescent plasma or monoclonal antibodies against SARS-CoV-2 among seronegative patients with COVID-19 requires further evaluation.
Illness severity associated with severe acute respiratory syndrome coronavirus (SARS-CoV-2) is unpredictable, ranging from asymptomatic infection to acute respiratory distress syndrome, multi-organ failure, and death (COVID-19).1 , 2 By April 2021, COVID-19 has claimed more than 2.8 million lives worldwide.3 Most proposed therapeutic strategies for COVID-19 have either targeted viral clearance or mitigating the excessive host inflammatory response associated with multi-organ failure and death.4
SARS-CoV-2-specific antibodies, particularly those preventing viral spike receptor-binding domain (RBD) interaction with the host angiotensin-converting enzyme 2 (ACE2) receptor, can neutralise the virus.5 The theoretical benefits of convalescent plasma in COVID-19 are supported by the association of its use during SARS coronavirus infection and a reduction in mortality, albeit limited to observational data.6 Any potential benefits conferred by convalescent plasma in COVID-19 disease therefore require evaluation.
We performed a systematic review, meta-analysis, and trial sequential analysis (TSA) of RCTs of convalescent plasma in the treatment of COVID-19. As convalescent plasma may be expected to provide most benefit in those at greatest risk of death, we also performed a meta-regression to investigate the relationship between treatment effect and overall risk. We further evaluated whether administration of convalescent plasma earlier in the disease course, or plasma containing higher titre antibodies, was associated with a mortality benefit.
Methods
This review was registered with the international Prospective Register of Systematic Reviews (PROSPERO registration number: CRD42021234201) and is reported adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary material).
Information sources and search strategy
PubMed, Embase, MedRxiv, and the Cochrane library were systematically searched using a controlled vocabulary (MeSH) and keywords without date or language restrictions. The last search update was on July 2, 2021. The Boolean search strategy was as follows: ((COVID-19 OR SARS-CoV-2) AND (convalescent plasma OR convalescent serum OR serotherapy OR passive immunization OR convalescence OR immunoglobulin OR IVIG OR antibody∗ OR monoclonal OR polyclonal OR recombinant) AND (clinical trials OR randomized trials OR randomised trials OR RCTs)). The control group was not defined in our search terms. Research papers and review articles were hand-searched for any further relevant trials.
Eligibility criteria
Inclusion and exclusion criteria were determined a priori. All trials comparing convalescent plasma or plasma products with either a placebo or standard care control group were considered. We included patients being treated with other COVID-19 therapies (co-interventions), details of which are provided in Supplementary material. Non-RCTs and paediatric populations were excluded.
Trial selection
Titles and abstracts were independently screened by two investigators (NS, TS) to exclude non-relevant trials with any discrepancies resolved by a third (NA). Any relevant full-text articles were retrieved and analysed for eligibility using the pre-defined inclusion criteria. The same authors performed subsequent data collection and analysis independently with discrepancies resolved by the same third author.
Data collection and analysis
Using a standardised data collection form, information was extracted from the selected trials. Data included country of trial, total number of participants, trial design, age of patients, number of patients admitted to ICU, number of patients requiring mechanical ventilation, noninvasive ventilation (NOV), or both, and number of patients who died. For patients in the treatment arm, details were collected on the timing of convalescent plasma therapy with regard to symptom onset, dose and duration of convalescent plasma, and antibody titre.
Primary and secondary outcomes
The primary outcome was mortality. Where available, 28 or 30 day mortality was analysed. Secondary outcomes included progression to severe disease defined as a requirement for mechanical ventilation or ICU admission. As convalescent plasma administration may be expected to provide most benefit in those at the greatest risk of death, we also performed a meta-regression to investigate the relationship between treatment effect and overall risk of death, as defined by the control group mortality. In addition, the effect on mortality of time from symptom onset to administration of convalescent plasma, and the level of neutralising antibody titre within administered convalescent plasma, were also assessed.
Subgroup analyses
To ascertain whether administration of convalescent plasma was associated with any clinical benefit after the onset of critical illness, we performed subgroup analysis on patients admitted to the ICU at time of enrolment, and on those patients receiving respiratory support at the time of trial enrolment.
Risk of bias assessment
The Cochrane Collaboration tool for assessing risk of bias (RoB2)7 was used to assess the methodological quality of the RCTs. This included the following domains: randomisation process, assignment to intervention, missing outcome data, measurement of outcome, selection of the reported result, other bias, and overall bias. The risk of bias in each domain was judged as low, high, or unclear.
Grading the quality of evidence
The Grading of Recommendation Assessment, Development, and Evaluation approach (GRADEpro Guideline Development Tool; McMaster University, 2015)8 was used to assess the quality of each outcome measure. The quality of evidence was downgraded on the basis of the following assessments: risk of bias, inconsistency, indirectness, imprecision, and other considerations. A funnel plot and Harbord's test were used to assess publication bias.9 The overall quality of evidence was subsequently rated as high, moderate, low, or very low.
Statistical analysis
ndividual trial data were combined for mortality using Mantel–Haenszel models with the reference group taken as the group randomised to standard care or placebo. The meta-analysis was performed using RevMan for Windows (version 5.1; Cochrane Collaboration, Oxford, UK). Statistical heterogeneity was assessed using the I 2 methodology. I 2 values ˃30%, >50%, and >75% indicated moderate, substantial, and considerable heterogeneity among trials, respectively. A random-effects model was used to analyse data. All P values were two-tailed and considered statistically significant if <0.05. Data on dichotomous outcomes are presented as odds ratio (OR), 95% confidence intervals (CI), P values; I 2 values. Meta-regression was performed using Stata version 16.1 (StataCorp, College Station, TX, USA).
TSA was performed using TSA programme version 0·9·5·10 (www.ctu.dk/tsa) as type 1 errors may occur in meta-analyses with sample sizes that are too small. TSA tests the credibility of the meta-analysis results by combining an estimate of the required information size (RIS) calculated from the cumulative sample size of included trials, with an adjusted threshold for statistical significance. Meta-analysis monitoring boundaries (Trial Sequential Monitoring Boundaries) and the RIS were quantified, alongside diversity adjusted information size (D 2) and adjusted 95% CIs. Diversity adjustment was performed according to an overall type I error of 5% and power of 80%. RIS was calculated using a relative risk reduction (RRR) of 31.5%, based on use of convalescent plasma in influenza A10 and the control event proportion obtained from our actual meta-analysis.
Protocol changes
The final protocol differed from the published PROSPERO protocol in the following ways: a random-effects model was used rather than a fixed-effects model owing to the number of studies identified, but included fixed effects as an additional sensitivity analysis. An additional sensitivity analysis was performed on trials in which the control group only received standard care. In addition to pre-defined primary and secondary outcomes, the odds of adverse events associated with the administration of convalescent plasma were also evaluated. Subgroup analysis was not performed on patients on respiratory support at enrolment as this information was not available. The RRR used for TSA analysis was incorrectly stated in the protocol as 26.6%; the correct RRR of 31.5% was therefore used instead.
Results
Search strategy
The search strategy identified 3493 articles. A total of 3093 articles remained after removal of duplicates and a further 3060 were excluded based on title alone, abstract alone, or both. Of the remaining 33 trials, 14 were excluded at full review; nine were non-randomised,11, 12, 13, 14, 15, 16, 17, 18, 19 three used a non-convalescent plasma product,20, 21, 22 one had an overlapping data set,23 and one randomised to early or late convalescent plasma.24 Two trials administered neutralising monoclonal antibodies.25 , 26 As there were no primary outcome events (mortality) in one of the two trials,25 we were unable to perform a meta-analysis on monoclonal antibodies in COVID-19. All analyses were therefore limited to the 17 trials that used convalescent plasma for COVID-19 disease27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 (Fig. 1 ).
Fig 1.
PRISMA flow chart. Flow chart of included and excluded trials. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Trial characteristics
Ten trials enrolled patients requiring advanced respiratory support including mechanical ventilation,29 , 31 , 32 , 36, 37, 38, 39, 40, 41, 42 Seven trials enrolled patients on NIV,31 , 32 , 39, 40, 41, 42, 43 and 12 trials enrolled patients on high flow nasal oxygen (HFNO)31, 32, 33, 34, 35, 36, 37 , 39, 40, 41, 42, 43 (Table 1 and Supplementary Table S1). Convalescent plasma was administered either as three doses on days 1, 3, and 5 in one study,42 two doses ranging from 200 to 250 ml30 , 33 12 h apart in one trial40 or 24 h apart in six trials,28 , 30 , 33, 34 , 38 , 41 or as a single dose ranging from 100 to 600 ml in six trials.27 , 29 , 31 , 32 , 39 , 43 Additional COVID-19 directed co-interventions used in the identified trials are listed in Supplementary Table S2. Those in the control group were administered a normal saline placebo in two trials,31 , 35 non-convalescent plasma in two trials,37 , 39 or intravenous immunoglobulin (IVIG) in one trial.41 The remaining 12 trials were open label. The 17 selected trials included 15 587 patients with 8027 (51.5%) allocated to the convalescent plasma arm and a mean weighted mortality of 25.1%.
Table 1.
Baseline characteristics of included trials. ChiCTR, Chinese clinical trial registry; CTRI, Clinical Trial Registry of India; HFO, high flow oxygen; NCT, National Clinical Trial registry; NIV, noninvasive ventilation; NS, not specified.
| Authors/trial registration | Country | Recruitment dates | Dose administered |
Numbers recruited |
Age (yr) |
Mechanical ventilation |
NIV |
HFO |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma | Control | Plasma | Control | Plasma | Control | Plasma | Control | Plasma | Control | Plasma | Control | |||
| Agarwal and colleagues (PLACID)30 CTRI: 2020/04/024775 |
India | April 22–July 14, 2020 | Two doses of 200 ml, 24 h apart | Open label | 235 | 229 | 52 ± 5 | 51 ± 5 | NS | NS | NS | NS | NS | NS |
| AlQahtani and colleagues34 NCT: 04356534 |
Bahrain | April–June 2020 | Two doses of 200 ml over 2 successive days | Open label | 20 | 20 | 53 ± 15 | 51 ± 13 | NS | NS | NS | NS | 3/20 (15%) |
1/20 (5%) |
| Avendaño and colleagues (ConPlas-19)27 NCT: 04345523 |
Spain | April 4–July 10, 2020 | Single dose of 250–300 ml | Open label | 38 | 43 | 61 ± 16 | 60 ± 15 | NS | NS | NS | NS | NS | NS |
| Bajpai and colleagues33 NCT: 04346446 |
India | April 21–May 30, 2020 | Two doses of 250 ml on consecutive days | Open label | 15 | 16 | 48 ± 9 | 48 ± 11 | NS | NS | NS | NS | 14/15 (93%) |
15/16 (94%) |
| Bennett-Guerrero and colleagues39 NCT: 04344535 |
United States | April 8–August 24, 2020 | Single dose of 2 units (∼480 ml) | Single dose of 2 units (approx. 480ml) | 59 | 15 | 67 ± 16 | 64 ± 17 | 11/59 (19%) |
3/15 (20%) |
3/59 (5%) |
2/15 (13%) |
Included in NIV | Included in NIV |
| Estcourt40 (REMAP-CAP) NCT: 02735707 |
Worldwide | March 9–January 18, 2021 | Two doses of 1 unit (∼550 ml) 12 h apart | Open label | 1078 | 909 | 60 ± 13 | 60 ± 13 | 356/1078 (33%) |
289/909 (32%) |
493/1078 (46%) |
407/909 (45%) |
225/1078 (21%) |
211/909 (23%) |
| Gharbharan and colleagues (ConCOVID)29 NCT: 04342182 |
The Netherlands | April 8–June 10, 2020 | Single dose of 300 ml | Open label | 43 | 43 | 54 ± 4 | 56 ± 5 | 13/43 (30%) |
NS | NS | NS | NS | NS |
| Gonzalez and colleagues41 NCT: 04381858 |
Mexico | May 5–October 17, 2020 | Two doses of 200 ml on consecutive days | Five doses of IVIG (0.3 g kg−1) on consecutive days | 130 | 60 | 61 ± 8 | 56 ± 6 | Included but NS | Included but NS | Included but NS | Included but NS | Included but NS | Included but NS |
| Horby and colleagues (RECOVERY)38 NCT: 04381936 |
UK | May 28, 2020–January 15, 2021 | Two doses of 275 ml on consecutive days | Open label | 5795 | 5763 | 64 ± 15 | 63 ± 15 | 302/5795 (5%) |
315/5763 (5%) |
NS | NS | NS | NS |
| Körper and colleagues (CAPSID)42 NCT: 04433910 |
Germany | August 30–December 24, 2020 | Three doses of 1 unit on days 1, 3, and 5 | Open-label | 53 | 52 | 59 ± 3 | 61 ± 3 | 13/53 (26%) |
17/52 (32%) |
28/53 (53%) |
21/52 (40%) |
Included in NIV | Included in NIV |
| Li and colleagues44 ChiCTR: 2000029757 |
China | February 14–April 1, 2020 | Single dose of 4–13 ml kg−1 | Open label | 52 | 52 | 71 ± 5 | 69 ± 4 | 14/51 (27%) |
11/50 (22%) |
21/51 (41%) |
23/50 (46%) |
21/51 (41%) |
23/50 (46%) |
| Libster and colleagues31 NCT: 04479163 |
Argentina | June 4–October 25, 2020 | Single dose of 250 ml | Normal saline | 80 | 80 | 76 ± 9 | 78 ± 8 | 2/80 (2.5%) |
4/80 (5%) |
1/80 (1.3%) |
6/80 (7.5%) |
1/80 (1.3%) |
6/80 (7.5%) |
| O'Donnell and colleagues37 NCT: 04359810 |
USA and Brazil | April 21–November 27, 2020 | Single dose of 200–250 ml | Single dose of 200–250ml non-convalescent plasma | 150 | 73 | 60 ± 7 | 62 ± 7 | 17/150 (11%) |
11/73 (15%) |
NS | NS | 125/150 (83%) |
57/73 (78%) |
| Rasheed and colleagues36 | Iraq | April 3–June 1, 2020 | Single dose of 400 ml | Open label | 21 | 28 | 56 ± 18 | 48 ± 15 | 17/21 (81%) |
22/28 (78.6%) |
NS | NS | 4/21 (19%) |
6/28 (21%) |
| Ray and colleagues28 CTRI: 2020/05/025209 |
India | May 31–October 12, 2020 | Two doses of 200 ml on 2 consecutive days. | Open label | 40 | 40 | 61 ± 12 | 61 ± 12 | NS | NS | NS | NS | NS | NS |
| Pouladzadeh and colleagues43 IRCT: 20200310046736N1 |
Iran | March–May 2020 | Single dose of 500 ml | Open label | 30 | 30 | 54 ± 10 | 57 ± 17 | 0 | 0 | 10/30 (33%) |
5/30 (17%) |
Included in NIV | Included in NIV |
| Simonovich and colleagues (PlasmAr)35 NCT: 04383535 |
Argentina | May 28–August 27, 2020 | Single dose of up to 500 ml | Normal saline | 228 | 106 | 63 ± 6 | 61 ± 6 | NS | NS | 0 | 0 | 11/228 (4.8%) |
7/106 (6.6%) |
Primary outcome
Mortality was defined at 2142 or 25 days31 in two trials and 28–30 days in the remaining trials. There was no evidence of a mortality benefit with convalescent plasma therapy compared with standard care (24.7% vs 25.5%; OR=0.94 [0.85–1.04]; P=0.23; I 2=4%; TSA adjusted CI, 0.84–1.05). The cumulative Z-curve crossed neither the conventional nor the TSA boundary for benefit or harm but did cross the boundary for futility having accrued more than the RIS cases (Table 2 and Fig. 2 ). At the time of reporting of mortality, 30.2% convalescent plasma group patients and 31.3% control group patients were still in hospital.
Table 2.
Primary, sub-group, and secondary outcome data for included trials. CI, confidence interval.
| Outcome | References | Intervention group | Control group | Conventional effect estimate [95% CI] | Overall effect | I2 (%) |
|---|---|---|---|---|---|---|
| Overall mortality | 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 | 1986/8027 (24.7%) | 1929/7560 (25.5%) | 0.94 [0.85–1.04] | Z=1.19, P=0.23 | 4 |
| ICU patient mortality | 33,37, 38, 39, 40,42 | 1673/6796 (24.6%) | 1641/6495 (25.3%) | 0.91 [0.75–1.09] | Z=1.04, P=0.30 | 39 |
| Disease progression | ||||||
| ICU admission | 31,35 | 69/308 (22.4%) | 31/185 (16.7%) | OR=0.80 [0.21–3.09] | Z=0.32, P=0.75 | 63 |
| Mechanical ventilation | 27,29, 30, 31, 32, 33, 34, 35, 36, 37, 38,40,43 | 1115/7105 (15.7%) | 1042/6771 (15.4%) | OR=1.01 [0.92–1.11] | Z=0.23, P=0.82 | 0 |
| Adverse events | ||||||
| Total | 27,28,31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 | 4324/7782 (55.6%) | 4136/7278 (56.8%) | OR=1.03 [0.80–1.34] | Z=0.26, P=0.80 | 28 |
| Allergic reactions | 28,30,32,33,35, 36, 37, 38, 39, 40, 41 | 214/7763 (2.8%) | 173/7293 (2.4%) | OR=1.18 [0.96–1.45] | Z=1.61, P=0.11 | 0 |
| Transfusion related cardiac overload | 27,28,30,37,38 | 131/6255 (2.1%) | 147/6147 (2.3%) | 0.88 [0.70–1.12] | Z=1.02, P=0.31 | 0 |
Fig 2.
Effect of convalescent plasma on mortality in included trials a. Forest plot of mortality in RCTs. Size of squares for odds ratio reflects weight of trial in pooled analysis. Horizontal bars represent 95% confidence intervals (CIs). (b) Trial sequential analysis of mortality in RCTs. Uppermost and lowermost curves represent trial sequential monitoring boundary lines for benefit and harm, respectively. Horizontal lines represent the traditional boundaries for statistical significance. Triangular lines represent the futility boundary. The cumulative Z-curve represents the trial data. A diversity-adjusted required information size (RIS) of 1522 was calculated using α=0.05 (two-sided), β=0.20 (power of 80%). Relative risk reduction of mortality reduction was 31.5%. The cumulative Z-curve crosses neither the conventional nor the TSA boundary for benefit or harm, but did cross the boundary for futility having exceeded the RIS. M–H, Mantel–Haenszel; TSA, trial sequential analysis.
Subgroup analysis
Six trials33 , 37, 38, 39, 40 , 42 reported mortality for patients admitted to the ICU at enrolment including 13 291 (51.1%) allocated to the treatment arm with a mean mortality of 24.9%. Convalescent plasma treatment was not associated with a mortality benefit in ICU patients (24.6% vs 25.3%; OR=0.91 [0.75–1.09]; P=0.31; I 2=39%).
Meta-regression
Meta-regression was used to assess the relationship between antibody titre and treatment effect. Six trials measured neutralising antibody titres27 , 30 , 37, 38, 39 , 42 and five trials measured immunoglobulin G (IgG) levels.28 , 31 , 32 , 35 , 36 There was no evidence of association between treatment effect (logOR) and log-concentration of neutralising antibodies (P=0.45; I 2=0%) or IgG (P=0.30; I 2=0%). In addition, there was no evidence of a relationship between treatment effect and time from symptom onset to administration of convalescent plasma and mortality (P=0.27; I 2=16%), or between treatment effect and risk of death and mortality (P=0.27; I 2=7%).
Sensitivity analyses
A sensitivity analysis performed on the primary outcome of 28–30 day mortality using a fixed-effects model revealed no mortality benefit with convalescent plasma therapy compared with standard care (24.7% vs 25.5%; OR=0.96 [0.89–1.03]; P=0.23; I 2=4%; TSA adjusted CI, 0.88–1.04).
An additional sensitivity analysis was performed excluding the three studies that administered either non-convalescent plasma37 , 39 or IVIG as control.41 Convalescent plasma was not associated with a mortality benefit (24.7% vs 25.4%; OR=0.96 [0.90–1.04]; P=0.35; I 2=0%; TSA adjusted CI, 0.89–1.04).
As the risk of bias was high in most trials, no additional analyses were performed on trials with a low risk of bias. A TSA sensitivity analysis was attempted using the RRR calculated from our meta-analysis of 3.0%; however, this could not be performed as only 8.4% of RIS cases had been accrued.
Secondary outcomes
Two trials reported incidence of ICU admission31 , 35 including 308 patients of whom 62.5% were allocated to the treatment group with a combined incidence of 14%. Convalescent plasma was not associated with a reduction in ICU admission compared with standard care (22.4% vs 16.7%; OR=0.80 [0.21–3.09]; P=0.75; I 2=63%; TSA adjusted CI, 0.0–196.05). The Z-curve crossed neither conventional or TSA boundary for benefit or harm, nor the futility boundary as only 5% of RIS cases had been accrued.
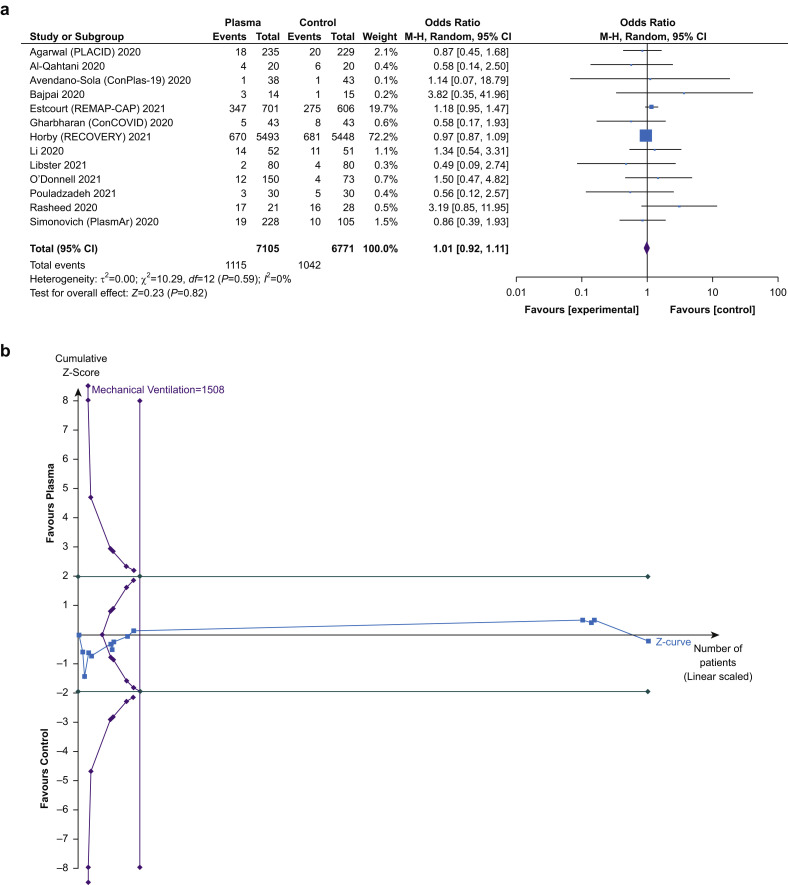
Thirteen trials reported the incidence of mechanical ventilation.27 , 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 , 40 , 43 These included 13 876 patients, of whom 7105 (51.2%) were allocated to the treatment group. Convalescent plasma was not associated with a reduction in need for mechanical ventilation (15.7% vs 15.4%; OR=1.01 [0.92–1.11]; P=0.82; I 2=0%; TSA adjusted CI, 0.91–1.13). The Z-curve crossed neither conventional nor TSA boundary for benefit or harm but did cross the boundary for futility having surpassed the RIS (Fig. 3 ).
Fig 3.
Effect of convalescent plasma on need for mechanical ventilation. (a) Forest plot of risk of need for mechanical ventilation. Size of squares for odds ratio reflects weight of trial in pooled analysis. Horizontal bars represent 95% confidence intervals. (b) Trial sequential analysis of risk of need for mechanical ventilation. Uppermost and lowermost curves represent trial sequential monitoring boundary lines for benefit and harm respectively. Horizontal lines represent the traditional boundaries for statistical significance. Triangular lines represent the futility boundary. M–H, Mantel–Haenszel.
Adverse events
Fifteen trials reported the incidence of total adverse events.27 , 28 , 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 These included a total population of 15 060 patients with 7782 (51.7%) allocated to the treatment arm and a combined incidence of 56.2%. Convalescent plasma administration was not associated with an increased rate of total adverse events compared with standard care (55.6% vs 56.8%; OR=1.03 [0.80–1.34]; P=0.80; I 2=28%; TSA adjusted CI, 0.72–1.50). The Z-curve crossed neither conventional or TSA boundary for benefit or harm but did cross the boundary for futility having exceeded the RIS (Supplementary Fig. S1). Additional adverse event analyses can be found in the Supplementary material.
Risk of bias and GRADE analysis
The risk of bias was high because of the open-label approach taken in 13 trials,27, 28, 29, 30 , 32, 33, 34 , 36 , 38 , 40, 41, 42, 43 industry sponsorship in 15 trials,27 , 28 , 30, 31, 32 , 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 and the release of results as non-peer-reviewed pre-prints by 11 trials,27, 28, 29 , 33 , 34 , 36, 37, 38 , 40, 41, 42 and thus was adjudged to be serious enough for GRADE analysis (Supplementary Table S3). Inconsistency was not serious excluding ‘Need for ICU admission’, which was deemed serious because of substantial heterogeneity. Indirectness was deemed not serious. Imprecision was judged as not serious in all domains excluding ‘Need for ICU admission’ as only 5% of RIS had been accrued. Some evidence of publication bias/small study effects was seen because of the asymmetry of the funnel plot (Harbord's test, P=0.010). The overall quality of evidence on GRADE assessment for our primary and secondary outcomes was marked as ‘very low’ (Table 3 and Supplementary Fig. S2).
Table 3.
GRADE analysis. GRADE, Grading of Recommendation Assessment, Development, and Evaluation; CI, confidence interval; OR, odds ratio; RIS, required information size. ∗Open label design. †Pre-print. ‡Asymmetrical funnel plot. ¶Positive Harbord's test. §Substantial heterogeneity. ||Only 5% RIS accrued.
| Certainty assessment |
No of patients |
Effect |
Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Convalescent plasma therapy | Standard care | Relative (95% CI) | Absolute (95% CI) | ||
| Mortality | ||||||||||||
| 17 | Randomised trials | Very serious∗,† | Not serious | Not serious | Not serious | Publication bias strongly suspected‡,¶ | 1986/8027 (24.7%) | 1929/7560 (25.5%) | OR 0.94 (0.85–1.04) |
12 fewer per 1000 (from 30 fewer to 8 more) |
 Very low |
Critical |
| Need for ICU admission | ||||||||||||
| 2 | Randomised trials | Not serious | Serious§ | Not serious | Very serious|| | Publication bias strongly suspected‡,¶ | 69/308 (22.4%) | 31/185 (16.8%) | OR 0.80 (0.21–3.09) |
29 fewer per 1000 (from 127 fewer to 216 more) |
 Very low |
Critical |
| Need for mechanical ventilation | ||||||||||||
| 13 | Randomised trials | Very serious∗,† | Not serious | Not serious | Not serious | Publication bias strongly suspected‡,¶ | 1151/7105 (16.2%) | 1042/6771 (15.4%) | OR 1.01 (0.92–1.11) |
1 more per 1000 (from 11 fewer to 14 more) |
 Very low |
Critical |
| Total adverse events | ||||||||||||
| 15 | Randomised trials | Very serious∗,†,‡ | Not serious | Not serious | Not serious | Publication bias strongly suspected‡,¶ | 4324/7782 (55.6%) | 4136/7278 (56.8%) | OR 1.03 (0.80–1.34) |
7 more per 1000 (from 55 fewer to 70 more) |
 Very low |
Critical |
Discussion
In patients with COVID-19, use of convalescent plasma was not associated with a mortality benefit. In patients with mild disease, convalescent plasma did not prevent either the need for mechanical ventilation nor ICU admission. A TSA suggests futility in continuing trial recruitment. Among patients with mild disease, convalescent plasma was not associated with a reduction in ICU admission or requirement for advanced respiratory support. No association was seen between the titre of anti-SARS-CoV-2 antibody infused, time from symptom onset to convalescent plasma administration, or risk of death and treatment effect of convalescent plasma.
Data on the significance of seroconversion on mortality in COVID-19 are conflicting. Levels of S- and RBD-specific IgG levels are higher in severe/critically ill patients during hospitalisation compared with patients with mild or moderate disease.44 , 45 At both early and late time points, plasma concentrations of IgA, IgG, and IgM antibodies are higher in survivors compared with those who subsequently die.46 In contrast, other studies suggest that the generation of S-, RBD-, and N-specific IgG occurs 1 week later in patients with severe/critically ill COVID-19 compared with those with mild/moderate disease, suggesting that early administration of convalescent plasma may benefit patients with more severe disease.44
The potential utility of endogenous anti-SARS-CoV-2 antibodies in overcoming acute infection with COVID-19 was supported by observational data. Early after symptom onset, levels of anti-N antibodies correlated strongly with disease severity.45 This may reflect illness severity, with greater antibody production in response to a greater antigen burden. We therefore hypothesised that administration of high-titre convalescent plasma may offer the greatest benefit and that anti-SARS-CoV-2 antibodies would have a beneficial effect on patients at greatest risk of death. However, meta-regression did not reveal any association between the risk of death and mortality benefit of convalescent plasma, nor any association between titre of convalescent plasma and mortality benefit.
Indeed, the concept of using convalescent plasma as a means of passive immunisation against COVID-19 was supported by early observational data, suggesting administration soon after hospitalisation using high-titre anti-spike protein RBD IgG significantly reduced mortality.47 We were however unable to find any association between timing of convalescent plasma administration with respect to symptom onset and effect on mortality.
None of the clinical trials stratified patients based on their levels of circulating anti-SARS-CoV-2 antibody titres before enrolment. A significant proportion of critically ill patients with COVID-19 generate high titres of anti-SARS-CoV-2 antibodies. The benefit of further augmenting this response through administration of convalescent plasma is questionable. It is not known whether early administration of high titre convalescent plasma could play a role in the management of high-risk patients, or in those with a progressively worsening illness trajectory, who lack endogenous anti-SARS-CoV-2 antibodies. Existing data suggest that administration of convalescent plasma is safe with no increase in adverse events; this provides reassurance for ongoing and future clinical trials.
We found significant heterogeneity between trials about convalescent plasma titres, doses, and timing of administration. These factors are likely to influence the efficacy of treatment. Furthermore, there is no standardised assay for measurement of neutralising antibodies, and different studies measured different antibodies against COVID-19, limiting the interpretation of impact of antibody titre on outcome. The data in this meta-analysis are heavily weighted by the RECOVERY trial,38 and interpretation of data is limited because of the high risk of bias in more than half of the trials. A significant number of patients enrolled in the trials had also received various co-interventions including antiviral medications, steroids, and other immunomodulators including tocilizumab. We were unable to correct for this and cannot exclude any interaction with convalescent plasma treatment. It was not possible to evaluate the effect of different dosing strategies on outcome. Nine trials permitted more than one dose of convalescent plasma therapy,28 , 30 , 33, 34, 35 , 38 , 40, 41, 42 but only two reported outcomes with respect to dose administration.40 , 41 Similarly, the reported incidence of allergic reactions, infections, and other complications varied significantly between trials. This may be attributable to differences in definitions, screening, reporting of complications, and variable patient follow-up. Although TSA suggests futility in ongoing trial recruitment, a smaller clinically relevant effect may still exist which would require further enrolment. Further trial data are required before firm conclusions can be made. This includes longer term outcomes as a proportion of patients remained as inpatients at the data censure cut point.
In summary, there was no clear benefit associated with convalescent plasma in COVID-19, with futility in continuing trial recruitment. No association was seen between the titre of anti-SARS-CoV-2 antibody infused, time from symptom onset to convalescent plasma administration, or risk of death and treatment effect of convalescent plasma. Early administration of high titre convalescent plasma to high-risk patients with a progressively worsening illness trajectory who lack endogenous anti-SARS-CoV-2 antibodies requires further attention, as does the use of monoclonal antibodies directed against SARS-CoV-2.
Authors' contributions
Study conception: NA
Literature search: NS, TS
Data extraction: NS, TS
Assessment of bias: TS, NS
Statistics: TS, GA, NS
Drafting manuscript: NS, NA, TS
Critical review: EN, LEM, MS
Finalising manuscript: all authors
Declarations of interest
MS reports grants and advisory board fees from NewB, grants from the Defence Science and Technology Laboratory, Critical Pressure, Apollo Therapeutics, advisory board and speaker fees (paid to his institution) from Amormed, Biotest, GE, Baxter, Roche, and Bayer, and honorarium for chairing a data monitoring and safety committee from Shionogi.
Author data access
All authors had access to data.
Handling editor: Jonathan Hardman
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2021.07.033.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.He J., Guo Y., Mao R., Zhang J. Proportion of asymptomatic coronavirus disease 2019: a systematic review and meta-analysis. J Med Virol. 2021;93:820–830. doi: 10.1002/jmv.26326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng Y., Liu W., Liu K., et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J. 2020;133:1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation . 2021. WHO coronavirus (COVID-19) dashboard.https://covid19.who.int/ Available from: [Google Scholar]
- 4.Snow T.A., Singer M., Arulkumaran N. Immunomodulators in COVID-19: two sides to every coin. Am J Respir Crit Care Med. 2020;202:1460–1462. doi: 10.1164/rccm.202008-3148LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roltgen K., Powell A.E., Wirz O.F., et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterne J.A.C., Savovic J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 8.Guyatt G.H., Oxman A.D., Vist G.E., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harbord R.M., Egger M., Sterne J.A. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 10.Beigel J.H., Aga E., Elie-Turenne M.-C., et al. Anti-influenza immune plasma for the treatment of patients with severe influenza A: a randomised, double-blind, phase 3 trial. Lancet Respir Med. 2019;7:941–950. doi: 10.1016/S2213-2600(19)30199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abolghasemi H., Eshghi P., Cheraghali A.M., et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfus Apher Sci. 2020;59:102875. doi: 10.1016/j.transci.2020.102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erkurt M.A., Sarici A., Berber I., et al. Life-saving effect of convalescent plasma treatment in covid-19 disease: clinical trial from eastern Anatolia. Transfus Apher Sci. 2020;59:102867. doi: 10.1016/j.transci.2020.102867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazitúa R., Briones J.L., Selman C., et al. Convalescent plasma in COVID-19. Mortality-safety first results of the Prospective Multicenter FALP 001-2020 trial. medRxiv. 2020 2020.11.30.20218560. [Google Scholar]
- 14.Ibrahim D., Dulipsingh L., Zapatka L., et al. Factors associated with good patient outcomes following convalescent plasma in COVID-19: a prospective phase II clinical trial. Infect Dis Ther. 2020;9:913–926. doi: 10.1007/s40121-020-00341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joyner M.J., Senefeld J.W., Klassen S.A., et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. medRxiv. 2020 Advance Access published on August 12. doi:2020.08.12.20169359. [Google Scholar]
- 16.AlShehry N., Zaidi S.Z.A., AlAskar A., et al. Safety and efficacy of convalescent plasma for severe COVID-19: interim report of a multicenter phase II study from Saudi Arabia. Saudi J Med Med Sci. 2021;9:16–23. doi: 10.4103/sjmms.sjmms_731_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salazar E., Perez K.K., Ashraf M., et al. Treatment of COVID-19 patients with convalescent plasma in Houston, Texas. medRxiv. 2020 Advance Access published on May 13. doi:2020.05.08.20095471. [Google Scholar]
- 18.Valentini R., Fernández J., Riveros D., et al. Convalescent plasma as potential therapy for severe COVID-19 pneumonia. medRxiv. 2020 Advance Access published on September 7. doi:2020.09.01.20184390. [Google Scholar]
- 19.Yoon H.A., Bartash R., Gendlina I., et al. Treatment of severe COVID-19 with convalescent plasma in the Bronx, NYC. JCI Insight. 2021;6 doi: 10.1172/jci.insight.142270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gharebaghi N., Nejadrahim R., Mousavi S.J., Sadat-Ebrahimi S.R., Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20:786. doi: 10.1186/s12879-020-05507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakoulas G., Geriak M., Kullar R., et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabarsi P., Barati S., Jamaati H., et al. Evaluating the effects of Intravenous Immunoglobulin (IVIg) on the management of severe COVID-19 cases: a randomized controlled trial. Int Immunopharmacol. 2021;90:107205. doi: 10.1016/j.intimp.2020.107205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen P., Nirula A., Heller B., et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with Covid-19. New Engl J Med. 2020;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balcells M.E., Rojas L., Le Corre N., et al. Early anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: a randomized phase II clinical trial. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottlieb R.L., Nirula A., Chen P., et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ACTIV-3/TICO LY-CoV555 Study Group A neutralizing monoclonal antibody for hospitalized patients with Covid-19. New Engl J Med. 2021;384:905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avendaño-Solà C., Ramos-Martínez A., Muñez-Rubio E., et al. A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia. J Clin Invest. 2021 doi: 10.1172/JCI152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ray Y., Paul S.R., Bandopadhyay P., et al. Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial. medRxiv. 2020 doi: 10.1101/2020.11.25.20237883. Advance Access published on November 29. [DOI] [Google Scholar]
- 29.Gharbharan A., Jordans C.C.E., Geurtsvankessel C., et al. Convalescent plasma for COVID-19. A randomized clinical trial. Nat Commun. 2021;12:3189. doi: 10.1038/s41467-021-23469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal A., Mukherjee A., Kumar G., et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Libster R., Perez Marc G., Wappner D., et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L., Zhang W., Hu Y., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bajpai M., Kumar S., Maheshwari A., et al. Efficacy of convalescent plasma therapy compared to fresh frozen plasma in severely ill COVID-19 patients: a pilot randomized controlled trial. medRxiv. 2020 doi: 10.1101/2020.10.25.20219337. Published online September 27. [DOI] [Google Scholar]
- 34.AlQahtani M., Abdulrahman A., Almadani A., et al. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID -19 disease. Sci Rep. 2021;11:9927. doi: 10.1038/s41598-021-89444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simonovich V.A., Burgos Pratx L.D., Scibona P., et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasheed A.M., Fatak D.F., Hashim H.A., et al. The therapeutic effectiveness of convalescent plasma therapy on treating COVID-19 patients residing in respiratory care units in Baghdad, Iraq. Le Infezioni in Medicina. 2020;28:357–366. [PubMed] [Google Scholar]
- 37.O’Donnell M.R., Grinsztejn B., Cummings M.J., et al. A randomized, double-blind, controlled trial of convalescent plasma in adults with severe COVID-19. J Clin Invest. 2021;131 doi: 10.1172/JCI150646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horby P.W., Estcourt L., Peto L., et al. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:2049–2059. doi: 10.1016/S0140-6736(21)00897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett-Guerrero E., Romeiser J.L., Talbot L.R., et al. Severe acute respiratory syndrome Coronavirus 2 convalescent plasma versus standard plasma in coronavirus disease 2019 infected hospitalized patients in New York: a double-blind tandomized trial. Crit Care Med. 2021;49:1015–1025. doi: 10.1097/CCM.0000000000005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The REMAP-CAP Investigators. Estcourt L.J. Convalescent plasma in critically ill patients with Covid-19. medRxiv. 2021 doi: 10.1101/2021.06.11.21258760. Advance Access published on June 13. [DOI] [Google Scholar]
- 41.Gonzalez J.L.B., González Gámez M., Mendoza Enciso E.A., et al. Efficacy and safety of convalescent plasma and intravenous immunoglobulin in critically ill COVID-19 patients. A controlled clinical trial. medRxiv. 2021 doi: 10.1101/2021.03.28.21254507. Advance Access published on March 31. [DOI] [Google Scholar]
- 42.Körper S., Weiss M., Zickler D., et al. Results of the CAPSID randomized trial for high-dose convalescent plasma in severe COVID-19 patients. J Clin Invest. 2021 doi: 10.1172/JCI152264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pouladzadeh M., Safdarian M., Eshghi P., et al. A randomized clinical trial evaluating the immunomodulatory effect of convalescent plasma on COVID-19-related cytokine storm. Intern Emerg Med. 2021:1–11. doi: 10.1007/s11739-021-02734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li K., Huang B., Wu M., et al. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat Commun. 2020;11:6044. doi: 10.1038/s41467-020-19943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashem A.M., Algaissi A., Almahboub S.A., et al. Early Humoral response correlates with disease severity and outcomes in COVID-19 patients. Viruses. 2020;12:1390. doi: 10.3390/v12121390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asif S., Frithiof R., Lipcsey M., et al. Weak anti-SARS-CoV-2 antibody response is associated with mortality in a Swedish cohort of COVID-19 patients in critical care. Crit Care. 2020;24:639. doi: 10.1186/s13054-020-03362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salazar E., Christensen P.A., Graviss E.A., et al. Significantly decreased mortality in a large cohort of coronavirus disease 2019 (COVID-19) patients transfused early with convalescent plasma containing high-titer anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein IgG. Am J Pathol. 2021;191:90–107. doi: 10.1016/j.ajpath.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.