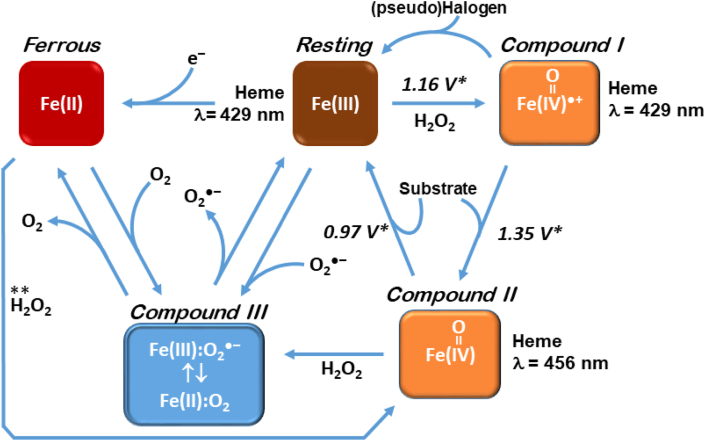
Fig. 4.
Different states of MPO and their associated redox potentials. The cycle begins with the “Resting” state, where the redox status of the iron centre of the protoporphyrin IX (haem) active site is indicated. MPO becomes activated in the presence of H2O2, which led to Compound I, which contains the ferryl haem species ([FeIV O]●+) and contains a delocalized free radical. Compound I will transform by either halogen oxidation (“pseudo halogen” is indicated to include SCN−, Cl−, Br−, I−) back to the Resting state, or the peroxidase cycle where xenobiotics and endobiotics (“Substrate”) can be oxidized, resulting in Compound II. In the presence of H2O2 and the absence of substrate, Compound III may form. Compound III can also form by certain substrates, or via reduction of the Resting enzyme to produce Ferrous MPO.
∗ Redox potentials from [95]. These values are useful in predicting if a particular substrate can reduce Compound I/II efficiently. As is apparent, Compound I is capable of oxidizing more substrates than Compound II. ** Indicates the direct conversion of ferrous myeloperoxidase to compound II by hydrogen peroxide [95].