Abstract
AIM
To explore retinal displacement after surgical treatment for idiopathic macular hole (IMH) with different internal limiting membrane (ILM) peeling patterns.
METHODS
Totally 22 eyes from 20 patients with IMH were randomly allocated into two groups, N-T group (11 eyes) and T-N group (11 eyes). For patients in N-T group, ILM was peeled off from nasal to temporal retina. For patients in T-N group, ILM was peeled off from temporal to nasal retina. Preoperative, postoperative 1, 3, and 6mo, autofluorescence fundus images were collected for manual measurement of distances of fixed nasal (N), temporal (T), superior (S), and inferior (I) retinal points (bifurcation or crossing of retinal vessels) around the macula to the optic disc (OD). These were respectively defined as N-OD, T-OD, S-OD, and I-OD. The retinal displacement, macular hole closure rate, and best corrected visual acuity (BCVA) were compared between the two groups after surgery.
RESULTS
At postoperative 1, 3, and 6mo, the macula slipped toward the OD, manifested by the decreased T-OD, N-OD, S-OD, and I-OD (P<0.05). No significant difference was found in the T-OD, N-OD, S-OD, and I-OD between N-T group and T-N group. IMH closure rate was 100% both in N-T group and T-N group. There was no significant difference in BCVA between two groups (P<0.05).
CONCLUSION
The macula slips toward the OD after successful macular hole surgery. The two different ILM peeling pattern show similar visual outcome and retinal displacement, which means ILM peeling directions are not the influencing factor of postoperative retinal displacement.
Keywords: retinal displacement, idiopathic macular hole, internal limiting membrane peeling, vitrectomy
INTRODUCTION
Macular hole is a retinal disorder located in the center of the fovea, threatening the vision of middle-aged and older patients[1]. Idiopathic macular hole (IMH) arises from vitreal traction on the macula in an either anteroposterior or tangential direction. Currently, vitrectomy has been used to improve visual dysfunction and metamorphopsia by releasing vitreofoveal traction[2]–[3].
Displacement of the retina is a new concept proposed by several scholars in recent years. Fundus autofluorescence (FAF) is a helpful technique for assessing unintentional retinal displacement after surgical treatment for eyes with rhegmatogenous retinal detachment, in which postoperative downward movement of the retina has been observed[4]–[5]. Before surgical treatment for epiretinal membrane (ERM), FAF photography demonstrated hyperautofluorescent lines, which indicated retinal displacement in 66.1% of patients[6].
It has been reported that the fovea moves to the optic disc (OD) after macular hole closure by a successful vitrectomy with internal limiting membrane (ILM) peeling[7]–[8]. Other investigations showed that successful surgical removal of ERM leads to significant retinal displacement[9]. However, there has not been a study that investigated the effect of different ILM peeling patterns on postoperative retinal displacement after successful closure of IMH.
This study aimed to explore unintentional retinal displacement after IMH surgery using FAF and analyze the effect of different ILM peeling patterns on the retinal displacement and visual function after the closure of IMH.
SUBJECTS AND METHODS
Ethical Approval
The study protocol is available at https://clinicaltrials.gov/ (ID NCT04655781). The study adhered to the tenets of the Declaration of Helsinki and was approved by the hospital Ethics Committee (approved No.2015-SR-191). Informed consent was signed by all participants before enrollment. Trial registration number: NCT04655781.
Subjects
Twenty-two eyes of 20 patients with IMH at the First Affiliated Hospital of Nanjing Medical University from January 2017 to January 2018 were recruited. Eyes excluded from this study were those with a history of macular hole surgery, high myopia, retinal detachment, proliferative vitreoretinopathy, or other macular disorders. The mean minimum linear diameter of macular hole was 536.00±234.21 µm for the N-T group and 571.64±251.90 µm for the T-N group, respectively (Table 1).
Table 1. Preoperative basic data.
| Group | Age (y) | Gender (F/M) | Left/right (n) | Macular hole duration (mo) | Macular hole MLD (µm) | Preop. BCVA (logMAR) | Lens opacity (yes/no) |
| N-T | 56.45±15.15 | 5/6 | 5/6 | 6.29±10.80 | 536.00±234.21 | 0.68±0.18 | 8/3 |
| T-N | 62.09±17.61 | 6/5 | 6/5 | 10.27±13.05 | 571.64±251.90 | 0.84±0.38 | 7/4 |
| P | 0.506 | 0.67 | 0.67 | 0.519 | 0.757 | 0.458 | 0.647 |
N-T: Nasal retina to temporal retina; T-N: Temporal retina to nasal retina; MLD: Minimum linear diameter; BCVA: Best-corrected visual acuity. P<0.05 statistically significant.
mean±SD
Surgical Techniques
Twenty-two eyes from 20 patients with IMH were randomly divided into the N-T group (11 eyes) and the T-N group (11 eyes). Patients were randomly assigned to either group using a table of random numbers by Hu ZZ in a 1:1 ratio. A standard vitrectomy with ILM peeling was carried out with 23- or 25-gauge (G) instruments in all participants. Two experienced surgeons (Xie P and Liu QH) performed the surgery. For patients in the N-T group, ILM peeling was performed from the nasal retina to the temporal retina (Figure 1, online supplementary Video 1). For patients in the the T-N group, ILM peeling was performed from the temporal retina to the nasal retina (Figure 1; online supplementary Video 2). After peeling of ILM and gas-fluid exchange, C3F8 was injected. Patients were immediately guided to maintain a facedown position.
Figure 1. Different internal limiting membrane peeling pattern.
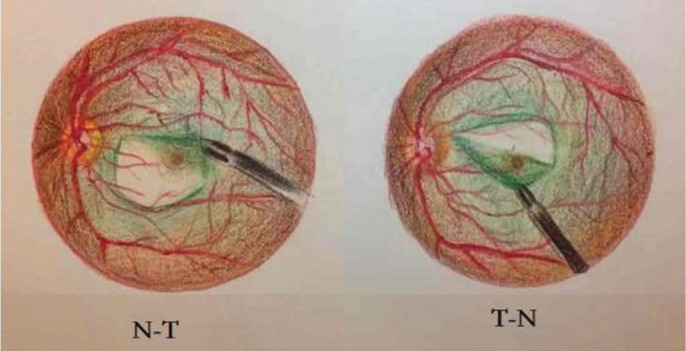
N-T: Nasal retina to temporal retina; T-N: Temporal retina to nasal retina.
Examinations
The primary outcome we measured was the retinal displacement. We investigated unintentional displacement of the retina by using FAF (HRA2, Heidelberg Engineering, Heidelberg, Germany). The macular hole size was measured by Cirrus optical coherence tomography (OCT; Zeiss Research Browser, version 6.0.2.81; Carl Zeiss Meditec, Inc; Figure 2). Measurement of retinal displacement is based on fixed points on the retina, such as turning points, bifurcations of retinal vessels, the crossing of vessels and starting point of the vessel at the temporal edge of the OD[9]. The locations of a bifurcation or junction of retinal vessels were marked in four regions of the macular area: nasal (N), temporal (T), superior (S), and inferior (I). The main indices of retinal displacement were distances of these fixed points to the starting point of the vessel at the temporal edge of the OD that are respectively defined as N-OD, T-OD, S-OD, and I-OD measured manually by FAF (Figure 2). Secondary outcomes were macular hole closure rate and the best-corrected visual acuity (BCVA). Measurement time points were preoperative, postoperative 1, 3, and 6mo. Two independent investigators were involved. The distances were measured manually on Image J software (https://imagej.net/) and averaged between the two investigators.
Figure 2. Retinal displacement measured by FAF and pre- and postoperative OCT images of macular hole.
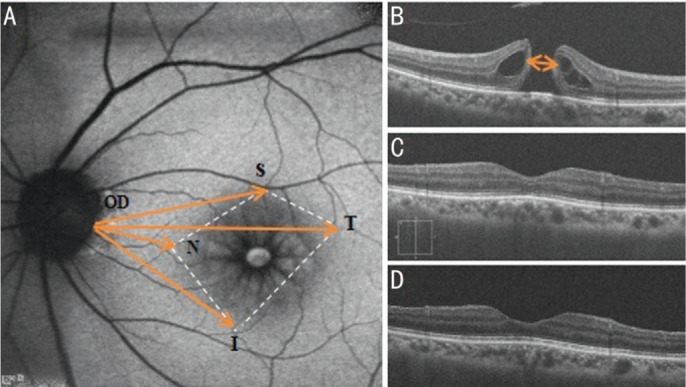
A: Retinal displacement measured by FAF. Distances of fixed nasal (N), temporal (T), superior (S), and inferior (I) retinal points to the starting point of the vessel at the temporal edge of the optic disc (OD) are respectively defined as N-OD, T-OD, S-OD, and I-OD. B: Preoperative OCT image; C: OCT image at postoperative 1mo; D: OCT image at postoperative 3mo. OCT: Optical coherence tomography.
Statistical Analysis
Statistical analyses of the data were carried out using SPSS software version 19.0 (SPSS, Inc., Chicago, IL, USA). Values were expressed as means±standard deviations. Chi-square test was used to determine baseline differences in gender, laterality of the eye, and lens opacity between two groups. The significance of the differences between pre- and postoperative values was also calculated by paired t-test. P<0.05 was considered statistically significant.
RESULTS
Basic Data
The preoperative baseline characteristics of all cases were summarized in Table 1. No statistically significant differences were found in age, gender, laterality of eyes, duration of macular holes, the minimum linear diameter of macular holes and preoperative BCVA (Table 1).
Postoperative Retinal Displacement
For all cases, displacement of the macular center toward the OD occurred at postoperative 1, 3, and 6mo, which was manifested by the decreased T-OD, N-OD, S-OD, I-OD.
There were statistically significant differences in N-OD, S-OD, I-OD between preoperative and postoperative 1mo. However, the difference in T-OD was not statistically significant (Table 2).
Table 2. Comparison of the retinal displacement before and after surgery.
| Time point | T-OD | S-OD | N-OD | I-OD |
| Preop. | 5.18±0.37 | 2.47±0.51 | 3.95±0.77 | 4.93±0.92 |
| 1mo postop. | 5.06±0.45 | 2.35±0.48 | 3.84±0.80 | 4.80±0.87 |
| P | 0.099 | 0.002 | 0.004 | 0.016 |
| Preop. | 5.18±0.37 | 2.47±0.51 | 3.95±0.77 | 4.93±0.92 |
| 3mo postop. | 5.03±0.45 | 2.31±0.47 | 3.85±0.83 | 4.78±0.90 |
| P | 0.047 | 0.003 | 0.003 | 0.006 |
| Preop. | 5.18±0.37 | 2.47±0.51 | 3.95±0.77 | 4.93±0.92 |
| 6mo postop. | 4.95±0.51 | 2.36±0.64 | 3.68±0.80 | 4.80±0.87 |
| P | 0.079 | 0.536 | 0.253 | 0.016 |
T-OD: Temporal vessel to the optic disc; N-OD: Nasal arcade to the optic disc; S-OD: Superior vessel to the optic disc; I-OD: Inferior vessel to the optic disc. Paired t-test. P<0.05 statistically significant.
mean±SD, mm
Differences in T-OD, N-OD, S-OD, I-OD between preoperative and postoperative 3mo were all statistically significant. Nevertheless, differences in T-OD, N-OD, S-OD, between preoperative and postoperative 6mo were not statistically significant except difference in I-OD (Table 2).
No statistically significant difference was found in T-OD, N-OD, S-OD, I-OD between the N-T group and the T-N group at preoperative, postoperative 1, 3, and 6mo (Table 3).
Table 3. Comparison of the retinal displacement between N-T group and T-N group.
| Time point | T-OD | N-OD | S-OD | I-OD |
| Preop. | ||||
| N-T | 5.14±0.29 | 2.36±0.59 | 3.90±0.65 | 4.75±0.80 |
| T-N | 5.21±0.46 | 2.59±0.40 | 4.00±0.90 | 5.12±1.03 |
| P | 0.634 | 0.350 | 0.788 | 0.345 |
| 1mo postop. | ||||
| N-T | 4.98±0.39 | 2.20±0.51 | 3.77±0.75 | 4.56±0.67 |
| T-N | 5.14±0.51 | 2.49±0.43 | 3.92±0.87 | 5.04±1.01 |
| P | 0.367 | 0.189 | 0.671 | 0.203 |
| 3mo postop. | ||||
| N-T | 4.91±0.40 | 2.13±0.48 | 3.77±0.80 | 4.58±0.71 |
| T-N | 5.15±0.47 | 2.50±0.36 | 3.93±0.90 | 4.99±1.05 |
| P | 0.103 | 0.062 | 0.681 | 0.279 |
| 6mo postop. | ||||
| N-T | 4.83±0.48 | 2.23±0.69 | 3.78±0.76 | 4.59±0.63 |
| T-N | 5.14±0.47 | 2.56±0.47 | 3.54±0.43 | 4.77±1.06 |
| P | 0.226 | 0.305 | 0.485 | 0.667 |
T-OD: Temporal vessel to the optic disc; N-OD: Nasal arcade to the optic disc; S-OD: Superior vessel to the optic disc; I-OD: Inferior vessel to the optic disc; N-T: Nasal retina to temporal retina; T-N: Temporal retina to nasal retina. Paired t-test. P<0.05 statistically significant.
mean±SD, mm
Postoperative Closing Rate of the Macular Hole
The closure rate of macular hole was 100% both in two groups. There was no significant difference in BCVA between the N-T group (0.49±0.24) and the T-N group (0.73±0.51) at the last follow-up (P=0.408).
DISCUSSION
IMH is a relatively common retinal disorder caused by adhesion in the vitreomacular interface[2]. Its formation is mainly related to vitreal traction on the vitreomacular interface in anteroposterior and tangential directions[10]–[14]. Surgical treatment for IMH includes removing the vitreous to release vitreofoveal traction and gas tamponade[15]. Successful closure of an IMH can be achieved by vitrectomy, especially with ILM peeling, which can eliminate tractional forces at the vitreomacular interface, hence improving functional recovery[7],[16]. Variations of the inverted ILM flap technique can successfully close large primary macular hole and significantly improve visual acuity. Successful secondary closure of recurrent macular hole can be achieved by early secondary vitrectomy with the extension of the ILM peeling[14]. It has been reported that the macula moves toward the OD after successful macular hole closure[17]–[18]. However, the mechanisms have not been fully clarified. Our results show that no significant difference was found in parameters of retinal displacement between different groups, indicating that tractional forces generated by ILM peeling does not affect postoperative retinal displacement. The differences in pre- and postoperative measurements of T-OD, N-OD, S-OD, I-OD were statistically significant at month 3 but not at month 6. The ‘rebound’ of the retinal displacement might be related to the traction of residual ILM after the closure of the macular hole.
ILM peeling has been widely applied in macular hole surgery, demonstrating higher macular hole closure rates and improved functional recovery, especially for stage 2 and stage 3 macular holes[16],[19]. It has been reported that a macula in which the ILM has been peeled off would slip toward the OD after surgical treatment for macular hole[7]–[8],[20]. Rodrigues et al[9] has reported that successful removal of ERM leads to significant retinal displacement. Our results show that displacement of the macular center toward the OD occurred at postoperative 1, 3, and 6mo, manifested by decreased T-OD, N-OD, S-OD, I-OD.
The mechanisms of retinal displacement after surgical treatment have not been fully clarified. Ishida et al[8] has reported that postoperative displacement of the temporal retina to the OD is greater than the nasal retina, suggesting that the temporal retina, which was retracting toward the OD during macular hole closure, is more flexible. Kawano et al[7] has found that for eyes in which macular hole spontaneously closed, there was no difference in distances of macular hole to OD and fovea to OD. Accordingly, the most probable cause of this movement turned out to be ILM peeling. Due to no ILM on the OD, the traction of the ILM on the fovea from the nasal side might be weaker than that from the temporal side under field conditions. The balance may be changed in case of ILM peeled, causing movement of the fovea to the OD. Our results were similar to that of Kawano et al[7]. There is no report to date about whether the traction of the ILM on the retina during ILM peeling will affect retinal displacement after pars plana vitrectomy. We explore for the first time whether ILM peeling patterns play roles in postoperative retinal displacement. Our results showed that different ILM peeling patterns yielded similar visual outcomes and retinal displacement, indicating that ILM peeling patternsare not the main influencing factor of postoperative retinal displacement.
In this study, we investigated unintentional displacement of the retina by using FAF. FAF can provide separate funduscopic images based on lipofuscin emissing light. The distribution pattern of fluorophore-containing lipofuscin determines the signal visualization[4]. FAF imaging can topographically map lipofuscin distribution in the retinal pigment epithelium (RPE) cell monolayer, the subneurosensory space and the outer retina[21]. FAF in the RPE depends on outer segment renewal and is influenced by a balance between clearance and accumulation. Thus, FAF could be considered as a clinical sign indicating RPE metabolic activity.
Nevertheless, we acknowledge that there are several limitations in this study. First, all macular holes were closed after surgery. The retinal displacement of patients with unclosed macular holes was not investigated. Second, since this work is a study with relatively limited cases, we did not make a stratified analysis. Third, we only investigated two different ILM peeling patterns in two directions. However, the surgeons can peel the ILM with varying grasp sites and different directions in clinical practice. Given that no statistically significant difference was found in two opposite ILM peeling directions, it might be unnecessary to investigate more ILM peeling directions. Fourth, metamorphopsia was not assessed in our study. It would be better to evaluate this parameter to explore the functional changes after surgical treatment. Further studies on a more significant number of participants with more extended follow-up periods are required to resolve the limitations.
In conclusion, we report for the first time that two different ILM peeling patterns showed similar visual outcomes and retinal displacement, indicating that ILM peeling directions are not the influencing factor of postoperative retinal displacement. In the future, we will explore the effects of the macular holes with different stages and different types, as well as different ILM peeling patterns on retinal displacement. Further investigation will help to provide more references for the pathogenesis and closure pattern of macular holes.
Acknowledgments
We thank all the MH patients who participated in our investigation and those who have helped us in the research but not mentioned as co-authors.
Foundations: Supported by the National Natural Science Foundation of China (No.81870669; No.81900875); Jiangsu Provincial Natural Science (No.BK20191059); Jiangsu Provincial Commission of Health and Family Planning (No.H201608).
Conflicts of Interest: Liu J, None; Hu ZZ, None; Zheng XH, None; Li YL, None; Huang JL, None; Cao EB, None; Yuan ST, None; Xie P, None; Liu QH, None.
REFERENCES
- 1.Bikbova G, Oshitari T, Baba T, Yamamoto S, Mori K. Pathogenesis and management of macular hole: review of current advances. J Ophthalmol. 2019;2019:3467381. doi: 10.1155/2019/3467381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh H. Idiopathic macular hole. Dev Ophthalmol. 2014;54:150–158. doi: 10.1159/000360461. [DOI] [PubMed] [Google Scholar]
- 3.Madi HA, Masri I, Steel DH. Optimal management of idiopathic macular holes. Clin Ophthalmol. 2016;10:97–116. doi: 10.2147/OPTH.S96090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiragami C, Shiraga F, Yamaji H, Fukuda K, Takagishi M, Morita M, Kishikami T. Unintentional displacement of the retina after standard vitrectomy for rhegmatogenous retinal detachment. Ophthalmology. 2010;117(1):86–92.e1. doi: 10.1016/j.ophtha.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Codenotti M, Fogliato G, Iuliano L, Querques G, Maestranzi G, Prati M, Ramoni A, De Benedetto U, Bandello F. Influence of intraocular tamponade on unintentional retinal displacement after vitrectomy for rhegmatogenous retinal detachment. Retina. 2013;33(2):349–355. doi: 10.1097/IAE.0b013e318263d180. [DOI] [PubMed] [Google Scholar]
- 6.Nitta E, Shiraga F, Shiragami C, Fukuda K, Yamashita A, Fujiwara A. Displacement of the retina and its recovery after vitrectomy in idiopathic epiretinal membrane. Am J Ophthalmol. 2013;155(6):1014–1020.e1. doi: 10.1016/j.ajo.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Kawano K, Ito Y, Kondo M, Ishikawa K, Kachi S, Ueno S, Iguchi Y, Terasaki H. Displacement of foveal area toward optic disc after macular hole surgery with internal limiting membrane peeling. Eye (Lond) 2013;27(7):871–877. doi: 10.1038/eye.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishida M, Ichikawa Y, Higashida R, Tsutsumi Y, Ishikawa A, Imamura Y. Retinal displacement toward optic disc after internal limiting membrane peeling for idiopathic macular hole. Am J Ophthalmol. 2014;157(5):971–977. doi: 10.1016/j.ajo.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues IA, Lee EJ, Williamson TH. Measurement of retinal displacement and Metamorphopsia after epiretinal membrane or macular hole surgery. Retina. 2016;36(4):695–702. doi: 10.1097/IAE.0000000000000768. [DOI] [PubMed] [Google Scholar]
- 10.Duker JS, Kaiser PK, Binder S, de Smet MD, Gaudric A, Reichel E, Sadda SR, Sebag J, Spaide RF, Stalmans P. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611–2619. doi: 10.1016/j.ophtha.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 11.Chung H, Byeon SH. New insights into the pathoanatomy of macular holes based on features of optical coherence tomography. Surv Ophthalmol. 2017;62(4):506–521. doi: 10.1016/j.survophthal.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Susvar P, Sood G. Current concepts of macular buckle in myopic traction maculopathy. Indian J Ophthalmol. 2018;66(12):1772–1784. doi: 10.4103/ijo.IJO_1126_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frisina R, Pilotto E, Midena E. Lamellar macular hole: state of the art. Ophthalmic Res. 2019;61(2):73–82. doi: 10.1159/000494687. [DOI] [PubMed] [Google Scholar]
- 14.Tam ALC, Yan P, Gan NY, Lam WC. The current surgical management of large, recurrent, or persistent macular holes. Retina. 2018;38(7):1263–1275. doi: 10.1097/IAE.0000000000002020. [DOI] [PubMed] [Google Scholar]
- 15.Morescalchi F, Costagliola C, Gambicorti E, Duse S, Romano MR, Semeraro F. Controversies over the role of internal limiting membrane peeling during vitrectomy in macular hole surgery. Surv Ophthalmol. 2017;62(1):58–69. doi: 10.1016/j.survophthal.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Hu Z, Ye X, Lv X, Liang K, Zhang W, Chen X, Cao E, Gu X, Liu Q, Liu Q, Xie P. Non-inverted pedicle internal limiting membrane transposition for large macular holes. Eye (Lond) 2018;32(9):1512–1518. doi: 10.1038/s41433-018-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi H, Inoue M, Koto T, Itoh Y, Hirota K, Hirakata A. Inverted internal limiting membrane flap technique for treatment of macular hole retinal detachment in highly myopic eyes. Retina. 2018;38(12):2317–2326. doi: 10.1097/IAE.0000000000001898. [DOI] [PubMed] [Google Scholar]
- 18.Chatziralli IP, Theodossiadis PG, Steel DHW. Internal limiting membrane peeling in macular hole surgery; why, when, and how? Retina. 2018;38(5):870–882. doi: 10.1097/IAE.0000000000001959. [DOI] [PubMed] [Google Scholar]
- 19.Yuan J, Zhang LL, Lu YJ, Han MY, Yu AH, Cai XJ. Vitrectomy with internal limiting membrane peeling versus inverted internal limiting membrane flap technique for macular hole-induced retinal detachment: a systematic review of literature and meta-analysis. BMC Ophthalmol. 2017;17(1):219. doi: 10.1186/s12886-017-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagomi T, Goto T, Tateno Y, Oshiro T, Iijima H. Macular slippage after macular hole surgery with internal limiting membrane peeling. Curr Eye Res. 2013;38(12):1255–1260. doi: 10.3109/02713683.2013.811261. [DOI] [PubMed] [Google Scholar]
- 21.Spaide R. Autofluorescence from the outer retina and subretinal space: hypothesis and review. Retina. 2008;28(1):5–35. doi: 10.1097/IAE.0b013e318158eca4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


