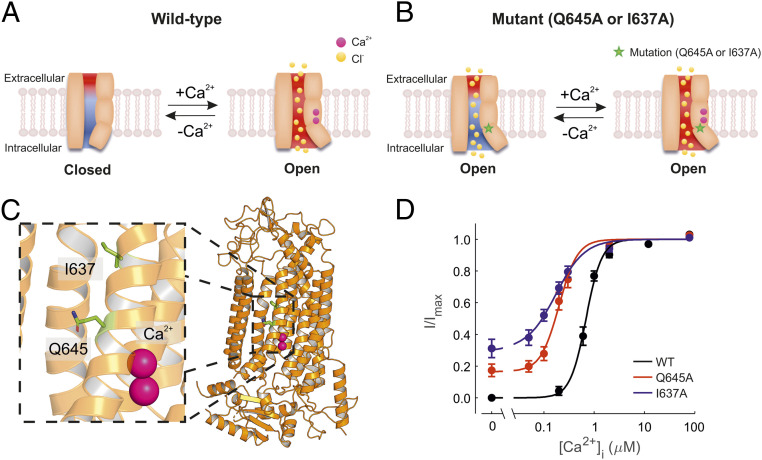
Fig. 1.
Ca2+ sensitivity of TMEM16A, TMEM16A-Q645A, and -I637A channels. (A) Diagrammatic representation of the TMEM16A channel pore and associated gating mechanisms based on previously published work (22, 24, 25, 31). The movement of the TM6 helix during gating is represented as a tilt on one side of the pore as the result of Ca2+ binding. The red and blue backgrounds depict negative and positive electrostatic potentials in the pore, respectively. The electrostatic gate is attenuated upon Ca2+ binding. (B) Diagrammatic representation of constitutively open channels (TMEM16A-Q645A and -I637A). The term “constitutively open” is used here to denote channels that are open in the absence of intracellular Ca2+ at positive Vm. Mutations are indicated by the star symbol. In these mutant channels, the electrostatic gate is presumably intact and can be attenuated upon Ca2+ binding. (C) The cryo-EM structure of TMEM16A with bound Ca2+ shown in pink (PDB ID: 5OYB). (D) Mean relationships between [Ca2+]i and the current measured at +70 mV for TMEM16A (n = 10), TMEM16A-Q645A (n = 7), and -I637A (n = 7), as indicated. The smooth curves are best fits of SI Appendix, Eq. S1 to the data.