Significance
Boronic acids are one of the most useful functional groups in organic chemistry and can be used as intermediates in synthesis or as key motifs in medicines. This work describes an extremely simple and economical way to use electrochemistry to convert carboxylic acids, through the intermediacy of redox-active esters, to boronic acids. The scope of this reaction is broad, the mechanism has been thoroughly studied, and it can be easily scaled up. Finally, application to the synthesis of a complex polycyclopropane natural product is demonstrated.
Keywords: electrochemistry, organic synthesis, total synthesis
Abstract
A simple electrochemically mediated method for the conversion of alkyl carboxylic acids to their borylated congeners is presented. This protocol features an undivided cell setup with inexpensive carbon-based electrodes and exhibits a broad substrate scope and scalability in both flow and batch reactors. The use of this method in challenging contexts is exemplified with a modular formal synthesis of jawsamycin, a natural product harboring five cyclopropane rings.
Boronic acids are among the most malleable functional groups in organic chemistry as they can be converted into almost any other functionality (1–3). Aside from these versatile interconversions, their use in the pharmaceutical industry is gaining traction, resulting in approved drugs such as Velcade, Ninlaro, and Vabomere (4). It has been shown that boronic acids can be rapidly installed from simple alkyl halides (5–19) or alkyl carboxylic acids through the intermediacy of redox-active esters (RAEs) (Fig. 1A) (20–24). Our laboratory has shown that both Ni (20) and Cu (21) can facilitate this reaction. Conversely, Aggarwal and coworkers (22) and Li and coworkers (23) demonstrated photochemical variations of the same transformation. While these state-of-the-art approaches provide complementary access to alkyl boronic acids, each one poses certain challenges. For example, the requirement of excess boron source and pyrophoric MeLi under Ni catalysis is not ideal. Although more cost-effective and operationally simple, Cu-catalyzed borylation conditions can be challenging on scale due to the heterogeneity resulting from the large excess of LiOH•H2O required. In addition to its limited scope, Li and coworkers’ protocol requires 4 equivalence of B2pin2 and an expensive Ir photocatalyst. The simplicity of Aggarwal and coworkers’ approach is appealing in this regard and represents an important precedent for the current study.
Fig. 1.
(A) Prior approaches to access alkyl boronic esters from activated acids. (B) Inspiration for initiating SET events electrochemically to achieve borylation. (C) Summary of this work.
At the heart of each method described above, the underlying mechanism relies on a single electron transfer (SET) event to promote decarboxylation and form an alkyl radical species. In parallel, the related borylation of aryl halides via a highly reactive aryl radical can also be promoted by SET. While numerous methods have demonstrated that light can trigger this mechanism (Fig. 1B) (16, 25–31), simple electrochemical cathodic reduction can elicit the same outcome (32–35). It was postulated that similar electrochemically driven reactivity could be translated to alkyl RAEs. The development of such a transformation would be highly enabling, as synthetic organic electrochemistry allows the direct addition or removal of electrons to a reaction, representing an incredibly efficient way to forge new bonds (36–40). This disclosure reports a mild, scalable, and operationally simple electrochemical decarboxylative borylation (Fig. 1C) not reliant on transition metals or stoichiometric reductants. In addition to mechanistic studies of this interesting transformation, applications to a variety of alkyl RAEs, comparison to known decarboxylative borylation methods, and a formal synthesis of the polycyclopropane natural product jawsamycin [(–)-FR-900848] are presented.
Results and Discussion
Initial experiments with RAE 1 were promising, delivering ca. 20% conversion to the borylated product. Extensive optimization of reaction conditions ultimately delivered 74% (64% isolated) yield of product 2 (Fig. 2B). While the breadth of this experimentation can be found in SI Appendix, outcomes from the screening of electron equivalents, solvent, electrolyte, current, electrode materials, and additives are showcased in Fig. 2A. Experimentally, 1.8 F/mol of RAE were needed to reach peak yields of 2; however, prolonged electrolysis resulted in erosion in yield due to observed product consumption (see SI Appendix).
Fig. 2.
(A) Graphical overview of electrochemical decarboxylative borylation optimization. Modifications listed as a function of yield. (B) Optimized reaction conditions.
Solvent had the most profound influence on the reaction, as a balance of conductivity, Lewis basicity, and polarity was needed to achieve high yields. In particular, the addition of N,N-dimethylformamide (DMF) (ε = 37) was vital, as it can act as a Lewis base with B2cat2 (the evidence of which can be seen by 11B NMR; SI Appendix) (22, 41) and undergo anodic oxidation (42), making the overall reaction redox neutral, obviating the need for a sacrificial anode. A combination of two inexpensive graphite electrodes gave yields close to those obtained using a sacrificial Zn anode.
The choice of electrolyte also impacted the reaction, with LiBr and LiBF4 facilitating the best outcomes. However, it should be noted that the addition of an electrolyte is not critical to the overall success of the reaction, as 61% yield of desired product 2 was observed by 1H NMR when electrolytes were excluded from the optimized reaction conditions (SI Appendix). Optimum results were observed when the reaction remained strictly anhydrous; therefore, LiBr was chosen as it is inexpensive and easily dried immediately prior to the reaction setup. On the 0.1-mmol scale used for optimization, a current >15 mA was required for high yields, with the added benefit that higher currents significantly shorten the reaction time. An optimum current density of 0.241 mA/mm2 was therefore identified, and holding this value constant was essential to achieving consistent yields when changing scale. The presence of any water during transesterification caused large inconsistencies in the yields, leading to a ∼20% loss in product. Therefore, pinacol was thoroughly azeotroped with toluene prior to use. Finally, a slight excess of B2cat2 (1.5 equivalents) was found to be necessary to achieve high yields.
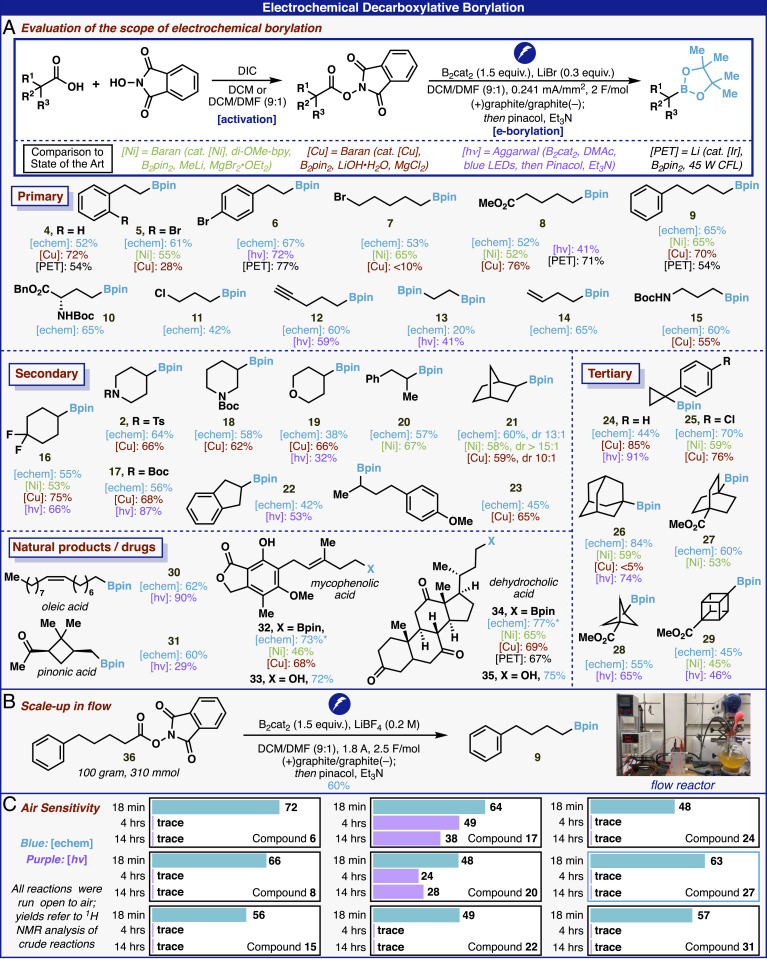
With optimized conditions in hand, the scope of this transformation was explored on a wide range of acids (Fig. 3A), often in yields equivalent to or higher than prior methods [comparative yields for Ni- (20), Cu- (21), hν- (22, 24), and photoinduced electron transfer– (23) based protocols are shown where reported]. This method proved highly chemoselective toward borylation of RAEs, demonstrating a broad functional group tolerance including both aryl and alkyl halides (5 to 7, 11, 16, and 25), ketones (31 and 34), esters (8, 10, and 27 to 29), alkenes (14, 30, and 32), alkynes (12), protected amines (2, 15, 17, and 18), and amino acids (10). RAEs derived from natural products and drug molecules smoothly underwent borylation, further highlighting the mildness of this method. Finally, bicyclic motifs commonly leveraged by medicinal chemists as phenyl bioisosteres (43) were smoothly borylated using the developed conditions (27 to 29). While this protocol proved successful on a range of substrates, there remained some limitations. In particular, conformationally flexible tertiary, benzylic, and α-heteroatom RAEs were nonproductive under these reaction conditions (SI Appendix).
Fig. 3.
(A and B) Scope and scale-up of the electrochemical decarboxylative borylation. See SI Appendix for experimental procedures. Yields refer to isolated yields of products from corresponding RAE. *Refers to NMR yield of products that were directly oxidized to corresponding alcohol prior to isolation. (C) Investigation of air sensitivity of electrochemical and photochemical borylation.
The protocol is highly scalable in an undivided cell and on smaller scales can be run using a commercial potentiostat. These conditions were easily translated to larger scales in batch using Erlenmeyer flasks, homemade electrodes, and a large direct current power supply (see Fig. 5C). Furthermore, the reaction was conducted on 100-g scale in a flow system, affording comparable yields to those obtained in batch (Fig. 3B). Additionally, these reaction conditions were remarkably tolerant to air (Fig. 3C); almost identical yields were obtained with the electrochemical conditions (open to air vs. under Ar). Alternatively, under air the photochemical variant of this transformation yields only trace product for most of the examined substrates. Furthermore, on a 0.13-mmol scale, the electrochemical reaction is complete in <20 min, which compares favorably to the >4 h required for the photochemical method (22). The robustness and short timeframe of this transformation make it an attractive alternative to the existing literature protocols.
Fig. 5.
(A) Formal synthesis of jawsamycin. (B) Examination of decarboxylative borylation conditions. (C) Scale-up of borylation. See SI Appendix for reagents and conditions.
Following the exploration of this method’s utility, preliminary studies were conducted to gain insight into the reaction mechanism. Cyclic voltammograms were performed to ascertain the identity of the species undergoing cathodic reduction (Fig. 4A). Interestingly, B2cat2 and the RAE independently exhibit reduction potentials in DMF at –2.98 V and –1.57 V, respectively (relative to Ag/AgCl). However, when a 3:2 ratio of B2cat2 and RAE is premixed in DMF a single reduction is observed at –1.96 V. This shift provides evidence for the existence of complex 37, initially proposed to be the active species under similar photochemical conditions (22). Further evidence for the existence of 37 was found through a series of 11B NMR experiments (SI Appendix).
Fig. 4.
(A–C) Experimental investigation of mechanism. (D) Proposed mechanistic picture.
Temporal measurements of reaction progress (Fig. 4B) reveal that the reaction exhibits zero-order kinetics in [B2cat2] and in [RAE] at higher concentrations (44-46). The slower rate at lower [RAE] may to be due to its consumption in unproductive reactions (N–O cleavage leading to acid and decarboxylation/protodeborylation). Zero-order kinetics in both substrates suggests that the RAE rapidly complexes with B2cat2 in solution to form species 37 prior to electron transfer, with the subsequent electron transfer at the cathode being rate-determining (first-order) (47, 48). The observation of positive rate dependence on current, a feature that we have observed in previous electrochemical reactions (49–51), supports the proposal that the transfer of electrons at the cathode is rate-limiting.
The presence of an intermediate alkyl radical was inferred by subjecting RAE 38 to the electrochemical borylation conditions (Fig. 4C). This resulted in a 9:1 ratio of linear (39) to cyclic (40) borylated products, arising from 5-exo trig cyclization of the ensuing radical (rate constant = 1.0 × 105 s−1) (52). Additionally, when RAE 41 (Fig. 4C) underwent electrochemical borylation only the homoallylic boronic ester 14 was isolated (rate constant = 1.3 × 108 s−1) (52).
Taken together, this evidence supports the mechanistic picture shown in Fig. 4D. To begin, DMF complexes with 1 equivalent of B2cat2 to form 42, which can coordinate with an RAE to form the ternary complex 37. This species then undergoes single-electron reduction in the rate-limiting step at the cathode, yielding 43. Rapid fragmentation of 43 results in the liberation of 1 equivalent of CO2, 44, and alkyl radical 45. This radical can then react with another equivalent of 42, yielding the desired alkyl boronic ester 46. The liberated boron-centered radical 47 is most likely quenched but in a minor pathway propagates a radical chain reaction by attacking a second RAE leading to 48, whose fragmentation would complete the cycle (see SI Appendix for further experimental investigation). This mechanistic picture closely mirrors Aggarwal and coworkers’ photochemical variant (22).
The kinetic results suggest a rationale for the enhanced efficiency of this electrochemical pathway with certain substrates. Generation of the free radical 45 from decarboxylation of 43 serves as entry into the cycle shown at the bottom of Fig. 4D, which can theoretically propagate by continuous regeneration of 45. However, in this scenario, the reaction at the cathode serves only to initiate this stand-alone cycle. For this scenario to hold, an induction period should be observed, as the slow step occurs off-cycle at the cathode, and it becomes difficult to rationalize the positive order dependence on current. More likely is that the dominant route to product 46 lies in the continual generation of radical 45 via cathodic reduction and the radical-chain cycle represents only a minor route (which cannot be ruled out as a productive pathway).
As a demonstration of the utility of this method, electrochemical borylation proved critical in accessing large quantities of key intermediates for a unique modular strategy en route to the polycyclopropane natural product jawsamycin [(–)-FR-900848, 57, Fig. 5A] (53, 54). All previous syntheses of this structurally fascinating natural product have commenced from olefins and relied on asymmetric cyclopropanations to build the polycyclopropane backbone (55–57). In contrast, this strategy represents a complimentary approach, hinging on the systematic coupling of chiral cyclopropane modules. This alternative retrosynthetic logic obviates the need for any asymmetric cyclopropanation step, as commercial building blocks such as 49 are easily desymmetrized to the corresponding hemiester, giving two differentiated synthetic handles which can be readily diversified through decarboxylative cross-coupling reactions (58).
At the outset, borylation of the chiral RAE 50 proved incredibly challenging using prior methods (Fig. 5B). Almost no product could be detected under Ni catalysis, and only modest yields were observed when employing either Cu or photochemical conditions. Even with the original optimized electrochemical protocol only ca. 13% of the desired alkyl boronic ester was formed, with the mass balance corresponding to either hydrolyzed acid or decarboxylated by-product. However, by utilizing electrodes with much higher surface areas (i.e., reticulated vitreous carbon) and activating the acid with the more electron-deficient tetrachloro N-hydroxyphthalimide derivative, workable yields of the desired product 51 were obtained (43% by NMR, 35% isolated). More importantly, this reaction was easily scaled (Fig. 5C), and up to 40 mmol of RAE could be processed in a single pass using a homemade batch reactor, with isolated yields up to 40% of the desired product 51.
With sufficient quantities of 51 in hand, the boronic ester underwent oxidative cleavage to the corresponding acid, and the stage was set to explore Pd-catalyzed oxidative homocoupling. To the best of our knowledge, the homodimerization of cyclopropyl-boronic acids/esters has not been reported before. Although multiple boronic esters were screened, only the acid was sufficiently active under these conditions. Performing the homocoupling under an atmosphere of oxygen to reoxidize Pd and complete the presumptive PdII/Pd0 catalytic cycle (59) proved critical to achieving reproducible results on scale (see SI Appendix for optimization summary). Ultimately, by using a Pd(OAc)2/dppf catalyst, K3PO4 as the base in THF/H2O, under a balloon of O2, the dimerization yielded 60% of 52, whose structure was confirmed by X-ray crystallography.
Next, to form the tetracyclopropane 54 this sequence of borylation and homocoupling was repeated. Mono saponification of dimer 52, followed by activation, and a second electrochemical borylation, afforded boronic ester 53. After oxidative cleavage to the boronic acid, however, we found that the original dimerization conditions resulted in no reaction; even on heating the reaction mixture to elevated temperatures only protodeborylated product was observed. After extensive optimization (SI Appendix), rigorous exclusion of water using a Cs2CO3/dioxane system, as well as utilizing the phosphine ligand P(o-Tol)3, began to result in product formation. The addition of AgOAc proved crucial here, ultimately corresponding to 54% of the desired tetramer 54. This dramatic improvement may arise from Ag serving dual roles by aiding the initial transmetalation of cyclopropyl boronic ester with Pd (60–65) as well as functioning as an external oxidant (66–70).
Mono saponification of the tetramer 54 and activation with TCNHPI set the stage for a final decarboxylative alkenylation with cyclopropyl vinyl-Bpin 55 (prepared in three steps; SI Appendix). Using a slight modification to previously reported Ni-catalyzed Suzuki couplings of RAEs (58), 45% of the desired vinylated product was obtained, which contained all five of the cyclopropane fragments of jawsamycin in place. A final reduction of the remaining ester completed the formal synthesis, intercepting the same strategic intermediate 56 used in both prior syntheses by Barrett and Kasdorf (55) and Falck et al. (56).
Conclusion
To summarize, a practical electrochemical decarboxylative borylation of alkyl RAEs has been developed. The scope and mechanism of this transformation are similar to the photochemical variant and can be conveniently scaled up in batch or flow (100 g). Finally, this newly developed method proved essential for the preparation of a key cyclopropane intermediate en route to the polycyclopropanated natural product jawsamycin.
Materials and Methods
All reagents were commercially available and used as supplied without further purification unless otherwise stated. The details of the materials and methods, including synthesis and characterization of substrates and intermediates in the synthesis of jawsamycin, are described in SI Appendix.
Supplementary Material
Acknowledgments
Financial support for this work was provided by the NIH (Grant GM-118176) and the NSF Center for Synthetic Organic Electrochemistry (Grant CHE-2002158). We are thankful to the NSF Graduate Research Fellowship Program (predoctoral fellowship to L.M.B.), Bristol Myers Squibb (graduate fellowship to L.M.B.), and the ARCS Foundation (graduate scholarship to L.M.B.). We are grateful to Dr. Dee-Hua Huang and Dr. Laura Pasternack (Scripps Research) for assistance with NMR spectroscopy; Prof. A. L. Rheingold, Dr. M. Gembicky, and Dr. J. B. Bailey (University of California San Diego) for X-ray crystallographic analysis; Dr. Jason Chen, Brittany Sanchez, and Emily Sturgell (Scripps Automated Synthesis Facility) for assistance with high-resolution mass spectrometry and liquid chromatography mass spectrometry; and Yinghua Xu and Chengpu Chu for supplying flow equipment and engineering expertise. We thank Dr. David Hill (Scripps Research) for assistance with kinetics experiments and useful discussions and Janice Dixon for assistance with manuscript preparation.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2109408118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Hall D. G., Ed., Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials (Wiley-VCH, Weinheim, Germany: ), ed. 2, 2011). [Google Scholar]
- 2.Sandford C., Aggarwal V. K., Stereospecific functionalizations and transformations of secondary and tertiary boronic esters. Chem. Commun. (Camb.) 53, 5481–5494 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Zhu C., Falck J. R., Transition-metal-free ipso-functionalization of arylboronic acids and derivatives. Adv. Synth. Catal. 356, 2395–2410 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plescia J., Moitessier N., Design and discovery of boronic acid drugs. Eur. J. Med. Chem. 195, 112270 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Yang C.-T., et al., Alkylboronic esters from copper-catalyzed borylation of primary and secondary alkyl halides and pseudohalides. Angew. Chem. Int. Ed. Engl. 51, 528–532 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Joshi-Pangu A., et al., Palladium-catalyzed borylation of primary alkyl bromides. J. Org. Chem. 77, 6629–6633 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yi J., et al., Alkylboronic esters from palladium- and nickel-catalyzed borylation of primary and secondary alkyl bromides. Adv. Synth. Catal. 354, 1685–1691 (2012). [Google Scholar]
- 8.Dudnik A. S., Fu G. C., Nickel-catalyzed coupling reactions of alkyl electrophiles, including unactivated tertiary halides, to generate carbon-boron bonds. J. Am. Chem. Soc. 134, 10693–10697 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito H., Kubota K., Copper(I)-catalyzed boryl substitution of unactivated alkyl halides. Org. Lett. 14, 890–893 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Bose S. K., Fucke K., Liu L., Steel P. G., Marder T. B., Zinc-catalyzed borylation of primary, secondary and tertiary alkyl halides with alkoxy diboron reagents at room temperature. Angew. Chem. Int. Ed. Engl. 53, 1799–1803 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Atack T. C., Lecker R. M., Cook S. P., Iron-catalyzed borylation of alkyl electrophiles. J. Am. Chem. Soc. 136, 9521–9523 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Liu M.-Y., Hong S.-B., Zhang W., Deng W., Expedient copper-catalyzed borylation reactions using amino acids as ligands. Chin. Chem. Lett. 26, 373–376 (2015). [Google Scholar]
- 13.Atack T. C., Cook S. P., Manganese-catalyzed borylation of unactivated alkyl chlorides. J. Am. Chem. Soc. 138, 6139–6142 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Bose S. K., et al., Highly efficient synthesis of alkylboronate esters via Cu(II)-catalyzed borylation of unactivated alky bromides and chlorides in air. ACS Catal. 6, 8332–8335 (2016). [Google Scholar]
- 15.Wang Z., Bachman S., Dudnik A. S., Fu G. C., Nickel-catalyzed enantioconvergent borylation of racemic secondary benzylic electrophiles. Angew. Chem. Int. Ed. Engl. 57, 14529–14532 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng Y., Mück-Lichtenfeld C., Studer A., Metal-free radical borylation of alkyl and aryl iodides. Angew. Chem. Int. Ed. Engl. 57, 16832–16836 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzarella D., Magagnano G., Schweitzer-Chaput B., Melchiorre P., Photochemical organocatalytic borylation of alkyl chlorides, bromides, and sulfonates. ACS Catal. 9, 5876–5880 (2019). [Google Scholar]
- 18.Liu Q., et al., Transition-metal-free borylation of alkyl iodides via a radical mechanism. Org. Lett. 21, 6597–6602 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Zhang L., Wu Z.-Q., Jiao L., Photoinduced radical borylation of alkyl bromides catalyzed by 4-phenylpyridine. Angew. Chem. Int. Ed. Engl. 59, 2095–2099 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Li C., et al., Decarboxylative borylation. Science 356, eaam7355 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J., et al., Cu-catalyzed decarboxylative borylation. ACS Catal. 8, 9537–9542 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fawcett A., et al., Photoinduced decarboxylative borylation of carboxylic acids. Science 357, 283–286 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Hu D., Wang L., Li P., Decarboxylative borylation of aliphatic esters under visible-light photoredox conditions. Org. Lett. 19, 2770–2773 (2017). [DOI] [PubMed] [Google Scholar]
- 24.VanHeyst M. D., et al., Continuous flow-enabled synthesis of bench-stable bicyclo[1.1.1]pentane trifluoroborate salts and their utilization in metallaphotoredox cross-couplings. Org. Lett. 22, 1648–1654 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Chen K., Wang L., Meng G., Li P., Recent advances in transition-metal-free aryl C–B bond formation. Synthesis 49, 4719–4730 (2017). [Google Scholar]
- 26.Mfuh A. M., Doyle J. D., Chhetri B., Arman H. D., Larionov O. V., Scalable, metal- and additive-free, photoinduced borylation of haloarenes and quaternary arylammonium salts. J. Am. Chem. Soc. 138, 2985–2988 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen K., Cheung M. S., Lin Z. Y., Li P. F., Metal-free borylation of electron-rich aryl (pseudo)halides under continuous-flow photolytic conditions. Org. Chem. Front. 3, 875–879 (2016). [Google Scholar]
- 28.Zhang L., Jiao L., Visible-light-induced organocatalytic borylation of aryl chlorides. J. Am. Chem. Soc. 141, 9124–9128 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Mfuh A. M., et al., Additive- and metal-free, predictably 1,2- and 1,3-regioselective, photoinduced dual C-H/C-X borylation of haloarenes. J. Am. Chem. Soc. 138, 8408–8411 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen K., Zhang S., He P., Li P., Efficient metal-free photochemical borylation of aryl halides under batch and continuous-flow conditions. Chem. Sci. (Camb.) 7, 3676–3680 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang M., Yang H., Fu H., Visible-light photoredox borylation of aryl halides and subsequent aerobic oxidative hydroxylation. Org. Lett. 18, 5248–5251 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Hong J. T., et al., Electrochemical radical borylation of aryl iodides. Chin. J. Chem. 37, 347–351 (2019). [Google Scholar]
- 33.Laza C., Duñach E., Serein-Spirau F., Moreau J. J. E., Vellutini L., Novel synthesis of arylboronic acids by electroreduction of aromatic halides in the presence of trialkyl borates. New J. Chem. 26, 373–375 (2002). [Google Scholar]
- 34.Laza C., Duñach E., New electrosynthesis of arylboronic esters from aromatic halides and pinacolborane. Adv. Synth. Catal. 345, 580–583 (2003). [Google Scholar]
- 35.Laza C., Pintaric C., Olivero S., Duñach E., Electrochemical reduction of polyhalogenated aryl derivatives in the presence of pinacolborane: Electrosynthesis of functionalised arylboronic esters. Electrochim. Acta 50, 4897–4901 (2005). [Google Scholar]
- 36.Yan M., Kawamata Y., Baran P. S., Synthetic organic electrochemical methods since 2000: On the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kingston C., et al., A survival guide for the “electro-curious”. Acc. Chem. Res. 53, 72–83 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollok D., Waldvogel S. R., Electro-organic synthesis—A 21st century technique. Chem. Sci. (Camb.) 11, 12386–12400 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Möhle S., et al., Modern electrochemical aspects for the synthesis of value-added organic products. Angew. Chem. Int. Ed. Engl. 57, 6018–6041 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiebe A., et al., Electrifying organic synthesis. Angew. Chem. Int. Ed. Engl. 57, 5594–5619 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hioe J., Karton A., Martin J. M. L., Zipse H., Borane-Lewis base complexes as homolytic hydrogen atom donors. Chemistry 16, 6861–6865 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Roth H. G., Romero N. A., Nicewicz D. A., Experimental and calculated electrochemical potentials of common organic molecules for applications to single-electron redox chemistry. Synlett 27, 714–723 (2016). [Google Scholar]
- 43.Mykhailiuk P. K., Saturated bioisosteres of benzene: Where to go next? Org. Biomol. Chem. 17, 2839–2849 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Blackmond D. G., Reaction progress kinetic analysis: A powerful methodology for mechanistic studies of complex catalytic reactions. Angew. Chem. Int. Ed. Engl. 44, 4302–4320 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Mathew J. S., et al., Investigations of Pd-catalyzed ArX coupling reactions informed by reaction progress kinetic analysis. J. Org. Chem. 71, 4711–4722 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Blackmond D. G., Kinetic profiling of catalytic organic reactions as a mechanistic tool. J. Am. Chem. Soc. 137, 10852–10866 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Burés J., A simple graphical method to determine the order in catalyst. Angew. Chem. Int. Ed. Engl. 55, 2028–2031 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nielsen C. D.-T., Burés J., Visual kinetic analysis. Chem. Sci. (Camb.) 10, 348–353 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu P., et al., Electroreductive olefin-ketone coupling. J. Am. Chem. Soc. 142, 20979–20986 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiang J., et al., Hindered dialkyl ether synthesis with electrogenerated carbocations. Nature 573, 398–402 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peters B. K., et al., Scalable and safe synthetic organic electroreduction inspired by Li-ion battery chemistry. Science 363, 838–845 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Griller D., Ingold K. U., Free-radical clocks. Acc. Chem. Res. 13, 317–323 (1980). [Google Scholar]
- 53.Yoshida M., et al., A novel antifungal antibiotic, FR-900848. I. Production, isolation, physico-chemical and biological properties. J. Antibiot. (Tokyo) 43, 748–754 (1990). [DOI] [PubMed] [Google Scholar]
- 54.Pietruszka J., Synthesis and properties of oligocyclopropyl-containing natural products and model compounds. Chem. Rev. 103, 1051–1070 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Barrett A. G. M., Kasdorf K., Total synthesis of the pentacyclopropane antifungal agent FR-900848. J. Am. Chem. Soc. 118, 11030–11037 (1996). [Google Scholar]
- 56.Falck J. R., Mekonnen B., Yu J. R., Lai J. Y., Synthesis of the polycyclopropane antibiotic FR-900848 via the Horeau gambit. J. Am. Chem. Soc. 118, 6096–6097 (1996). [Google Scholar]
- 57.Verbicky C. A., Zercher C. K., Olefin cross-metathesis in the preparation of polycyclopropanes: Formal synthesis of FR-900848. Tetrahedron Lett. 41, 8723–8727 (2000). [Google Scholar]
- 58.Chen T. G., et al., Building C(sp3)-rich complexity by combining cycloaddition and C-C cross-coupling reactions. Nature 560, 350–354 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adamo C., Amatore C., Ciofini I., Jutand A., Lakmini H., Mechanism of the palladium-catalyzed homocoupling of arylboronic acids: Key involvement of a palladium peroxo complex. J. Am. Chem. Soc. 128, 6829–6836 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Weibel J. M., Blanc A., Pale P., Ag-mediated reactions: Coupling and heterocyclization reactions. Chem. Rev. 108, 3149–3173 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Lennox A. J. J., Lloyd-Jones G. C., Selection of boron reagents for Suzuki-Miyaura coupling. Chem. Soc. Rev. 43, 412–443 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Zou G., Reddy Y. K., Falck J. R., Ag(I)-promoted Suzuki-Miyaura cross-couplings of n-alkylboronic acids. Tetrahedron Lett. 42, 7213–7215 (2001). [Google Scholar]
- 63.Chen J., Cammers-Goodwin A., 2-(Fluorophenyl)pyridines by the Suzuki-Miyaura method: Ag2O accelerates coupling over undesired ipso substitution (SNAr) of fluorine. Tetrahedron Lett. 44, 1503–1506 (2003). [Google Scholar]
- 64.Korenaga T., Kosaki T., Fukumura R., Ema T., Sakai T., Suzuki-Miyaura coupling reaction using pentafluorophenylboronic acid. Org. Lett. 7, 4915–4917 (2005). [DOI] [PubMed] [Google Scholar]
- 65.Chen H., Deng M. Z., Silver oxide mediated palladium-catalyzed cross-coupling reaction of cyclopropylboronic acids with allylic bromides. J. Org. Chem. 65, 4444–4446 (2000). [DOI] [PubMed] [Google Scholar]
- 66.Funes-Ardoiz I., Maseras F., Oxidative coupling mechanisms: Current state of understanding. ACS Catal. 8, 1161–1172 (2018). [Google Scholar]
- 67.Stuart D. R., Villemure E., Fagnou K., Elements of regiocontrol in palladium-catalyzed oxidative arene cross-coupling. J. Am. Chem. Soc. 129, 12072–12073 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Zhao J., Huang L., Cheng K., Zhang Y., Palladium-catalyzed alkenation of thiophenes and furans by regioselective C–H bond functionalization. Tetrahedron Lett. 50, 2758–2761 (2009). [Google Scholar]
- 69.Wen P., et al., Palladium-catalyzed C-2 selective C–H olefination of pyridines. Adv. Synth. Catal. 354, 2135–2140 (2012). [Google Scholar]
- 70.Zhao B., Pd-catalyzed C-3 functionalization of indolizines via C-H bond cleavage. Org. Biomol. Chem. 10, 7108–7119 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.