Fig. 1.
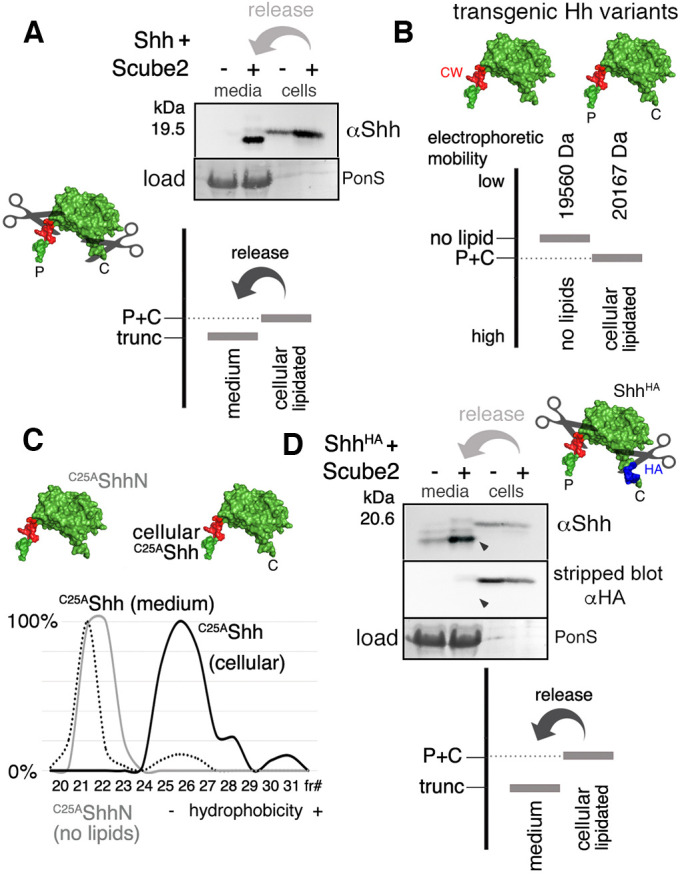
Scube2 enhances proteolytic Shh processing. (A–D) Schematics of expressed Shh constructs are shown, using PDB:3M1N as a template. P, palmitate; C, cholesterol; CW, Cardin–Weintraub motif representing the N-terminal cleavage site; scissors indicate loss of lipidated terminal peptides during release from Bosc23 cells. CW is shown in red; an inserted HA tag is shown in blue. (A) Increased electrophoretic mobility of soluble Shh (trunc) over the dual-lipidated cellular protein (P+C) results from the loss of both lipidated terminal peptides during release from Bosc23 cells (indicated by scissors), and this process depends on Scube2. Cellular (cells) and released (media) proteins were detected using Shh-specific antibodies (αShh). Residual serum albumin in TCA-precipitated supernatants served as loading control (PonceauS staining, PonS). Bottom: schematic of Shh release. (B) According to previous publications (Pepinsky et al., 1998; Porter et al., 1996), dual-lipidated Hh (cellular lipidated) migrates faster in SDS–PAGE than E. coli-expressed unlipidated Hh (no lipids), although mass spectrometry has determined molecular masses of 20,167 Da for the former form and 19,560 Da for the latter. Increased electrophoretic lipidated Hh mobility, despite higher molecular mass, is caused by SDS association with the large hydrophobic sterol backbone of cholesterol and the C16 hydrocarbon tail of the palmitate. Consistent with this, chemical hydrolysis of the ester bond that attaches cholesterol to Shh decreases the electrophoretic mobility of the delipidated product (Zeng et al., 2001). The observed increase in dual-lipidated Shh electrophoretic mobility during release from human cells (as shown in A) can therefore only result from the additional loss of associated terminal peptides, which more than compensates for this decrease. (C) Reverse-phase HPLC. The elution profile of Bosc23-expressed non-lipidated control C25AShhN (gray line) resembles that of soluble C25AShh (dotted line), but not that of its lipidated cellular precursor (black solid line). Elution profiles are expressed relative to the highest protein amount in a given fraction (set to 100%). fr#, fraction number. (D) Insertion of a C-terminal HA tag supports shedding. Removal of terminal peptides including the 1 kDa C-terminal tag increases the net electrophoretic mobility gain of solubilized proteins (arrowhead) (Jakobs et al., 2014, 2017), as visualized using antibodies against Shh and the HA tag (αHA). Data in A,C,D are representative of at least three independent experiments.
