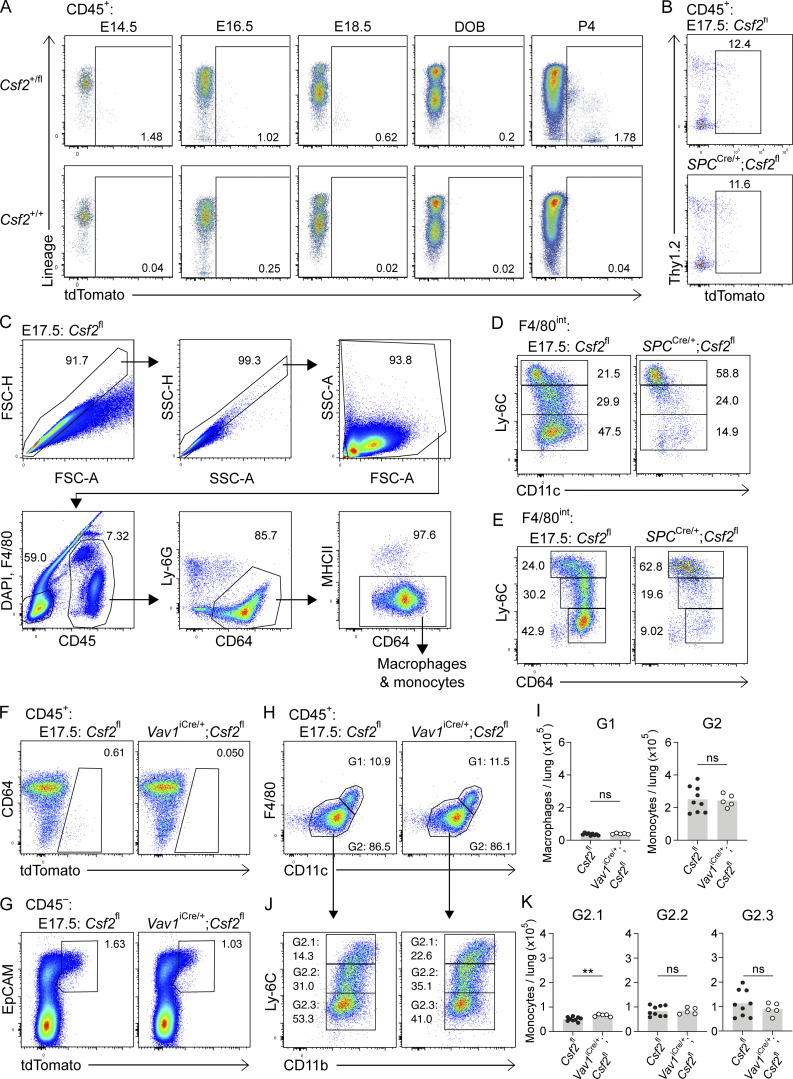
Figure S4.
Hematopoietic-derived GM-CSF is not critical for AM fate specification during embryogenesis. (A) Flow cytometry analysis of kinetics of tdTomato expression in perinatal lungs in Csf2+/fl and Csf2+/+ mice ranging from E14.5 to P4, gated on CD45+ cells. DOB, day of birth. (B–E) Analysis of E17.5 lungs isolated from Csf2fl and SPCCre/+;Csf2fl mice. (B) Flow cytometry analysis of tdTomato+ population, gated on CD45+ cells. (C) Gating strategy used to identify fetal macrophage and monocyte populations. (D) Flow cytometry analysis of Ly-6C and CD11c levels in the developing fetal monocyte population (G2 in Fig. 5 D). (E) Flow cytometry analysis of Ly-6C and CD64 levels in the developing fetal monocytes population (G2 in Fig. 5 D). (F–J) Analysis of E17.5 lungs isolated from Csf2fl and Vav1iCre/+;Csf2fl mice. (F) Flow cytometry analysis of the tdTomato+ population, gated on CD45+ cells. (G) Flow cytometry analysis of the EpCAM+tdTomato+ population, gated on CD45− cells. (H) Flow cytometry analysis of F4/80high primitive macrophages (G1) and F4/80int fetal monocytes (G2), gated on CD45+Ly-6G−CD64+MHCII− cells. (I) Quantification of primitive macrophages (G1) and fetal monocytes (G2). (J) Flow cytometry analysis of Ly-6C and CD11b levels in the developing fetal monocytes population (G2). (K) Quantification of Ly-6Chigh (G2.1), Ly-6Cint (G2.2), and Ly-6Clow (G2.3) fetal monocytes. (A) Data are representative of at least two independent experiments per time point. (B–H and J) Data are from one experiment representative of three (B–E) or two (F–H and J) independent experiments. (I and K) Data are pooled from two independent experiments. ns, P ≥ 0.05; **, P 0.01–0.001.