Figure 3.
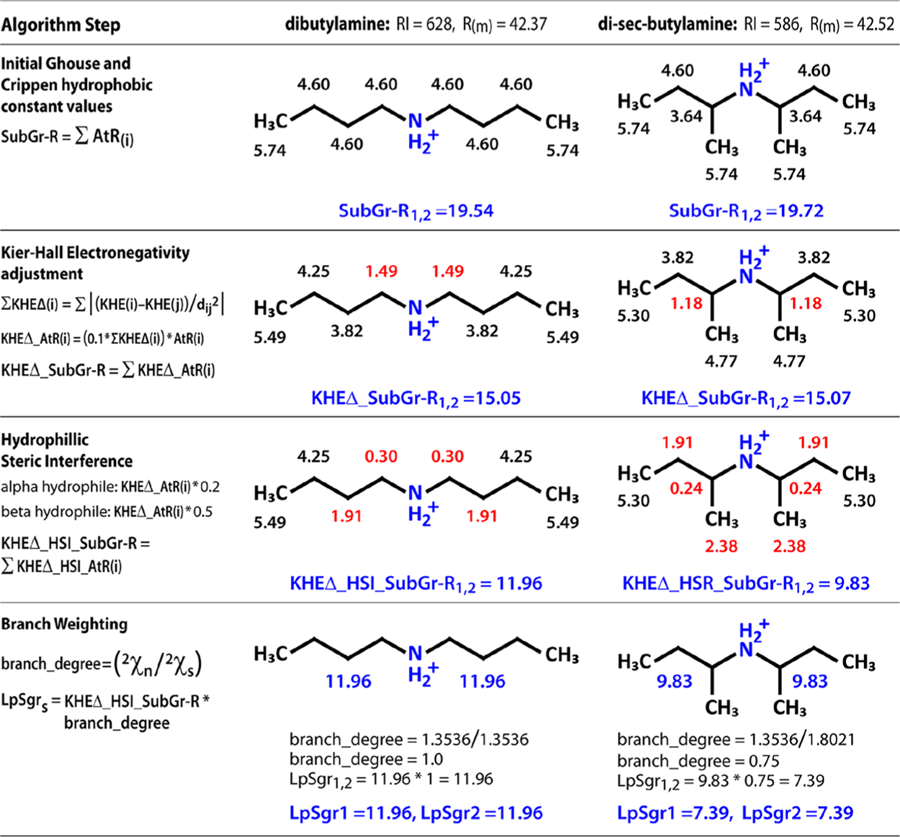
Calculating lipophilic subgraph descriptors for dibutylamine and di-sec-butylamine. Step 1 assigns atom-level refraction (AtR) values based on the Gouse Crippen approximation. Step 2 adjusts AtR by summing the Kier−Hall electronegativity difference against all other atoms in the molecule and multiplying AtR by a scaling factor to create KHEΔ_AtR(i) for each atom. The third step adjusts KHEΔ_AtR by multiplying an interference coefficient based on the count of alpha and beta hydrophilic atoms to create KHEΔ_HSI_AtR(i). Finally, KHEΔ_HSI_AtR(i) for each atom in the subgraph is summed and multiplied by a branch degree coefficient. The series of four steps results in descriptor values that reflect the difference in RI value where molar refraction does not. Intermediate subgraph sum values are given for each step to illustrate.
