Abstract
Interstitial lung disease (ILD) is a relatively frequent manifestation of systemic autoimmune rheumatic disorders (SARDs), including systemic sclerosis (SSc), rheumatoid arthritis (RA), idiopathic inflammatory myopathies (IIM), systemic lupus erythematosus (SLE), primary Sjögren’s syndrome (pSS), and anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis. Interstitial pneumonia with autoimmune features (IPAF) has been proposed to describe patients with ILD who have clinical or serological findings compatible with SARDs but they are not sufficient for a definite diagnosis. ILD may present with different patterns among patients with SARDs, but most commonly as nonspecific interstitial pneumonia (NSIP), with the exception of RA and ANCA vasculitis that more often present with usual interstitial pneumonia (UIP). The natural history of ILD is quite variable, even among patients with the same SARD. It may present with subclinical features following a slow progressively course or with acute manifestations and clinically significant rapid progression leading to severe deterioration of pulmonary function and respiratory failure. The radiographic pattern of ILD, the extent of the disease, the baseline pulmonary function, the pulmonary function deterioration rate over time and clinical variables related to the primary SARD, such as age, sex and the clinical phenotype, are considered prognostic factors for SARDs-ILD associated with adverse outcomes and increased mortality. Different modalities can be employed for ILD detection including clinical evaluation, pulmonary function tests, high resolution computed tomography and novel techniques such as lung ultrasound and serum biomarkers. ILD may determine the clinical outcome of SARDs, since it is associated with significant morbidity and mortality and therefore screening of patients with SARDs for ILD is of great clinical importance.
Keywords: anti-synthetase syndrome, dermatomyositis, interstitial lung disease, interstitial pneumonia with autoimmune features, natural history, polymyositis, rheumatoid arthritis, screening, Sjögren’s syndrome, systemic lupus erythematosus, systemic sclerosis, vasculitis
Introduction
Lung involvement is a rather common manifestation of systemic autoimmune rheumatic disorders (SARDs) contributing substantially to the morbidity and mortality. Interstitial lung disease (ILD) is characterized by diffuse parenchymal lung injury and is considered a serious pulmonary complication with significant impact on morbidity and mortality of patients with SARDs.1 SARDs related ILD include systemic sclerosis (SSc), dermatomyositis (DM), polymyositis (PM), rheumatoid arthritis (RA), primary Sjögren’s syndrome (pSS), mixed connective tissue disease (MCTD), systemic lupus erythematosus (SLE), and anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV). The prevalence of ILD in SARDs varies widely according to different studies. Systemic sclerosis is the SARD which is most commonly associated with interstitial lung disease, followed by idiopathic inflammatory myopathies including antisynthetase syndrome, mixed connective tissue disease and rheumatoid arthritis.2 Interstitial pneumonia with autoimmune features (IPAF) is a term that has been recently proposed for the subset of patients with ILD and clinical or serological features consistent with an SARD, who do not meet any diagnostic criteria.3 The prevalence and incidence of IPAF in the general population remains unclear and further studies are required to address this issue.4 Although IPAF is a clinically oriented term, it is mainly used for research purposes. Apart from IPAF, it is noteworthy, that a proportion of patients suffering from different types of SARDs-ILD, demonstrate a progressive clinical course, similar to that of idiopathic pulmonary fibrosis (IPF), with deterioration of pulmonary function and extension of lung fibrosis, implying common pathogenetic mechanisms and clinical outcome as a shared phenotypic feature. The term progressive fibrosing ILD (PF-ILD) has been proposed for this particular subset of SARDs-ILD patients.5,6
The histopathologic and imaging classification of SARDs-ILD follows that of idiopathic interstitial pneumonias (IIPs) and includes usual interstitial pneumonia (UIP), nonspecific interstitial pneumonia (NSIP), cryptogenic organizing pneumonia (COP), acute interstitial pneumonia (AIP) and lymphoid interstitial pneumonia (LIP).7 The histological pattern of UIP is characterized by heterogeneity with areas of normal lung tissue, interstitial inflammation, fibroblast foci and honeycomb changes, which correlate with radiological findings on high resolution computed tomography (HRCT) of bibasilar subpleural reticular opacities, honeycombing, traction bronchiectasis and less prominently, ground glass opacities.8 NSIP is characterized by a more homogeneous histologic appearance with interstitial infiltration by mononuclear cells and fibrosis of various degree, while HRCT imaging shows ground-glass and reticular opacities.9 The clinical course of SARD-ILD is often insidious with slow progression, but acute forms are also seen in clinical practice depending on the histologic subtype and the underlying SARDs. ILD in systemic autoimmune rheumatic diseases may determine the final clinical outcome of patients.10 Given the availability of targeted therapies, early diagnosis of SARDs-ILD is of great clinical importance. In this review, we focus on the natural history and screening of patients with systemic autoimmune rheumatic diseases and ILD.
Search strategy and selection criteria
We searched PubMed for randomized controlled trials, observational studies, meta-analyses and systematic reviews published between 1995 and March 2021, using combination of terms including “interstitial lung disease,” “rheumatoid arthritis,” “systemic sclerosis,” “dermatomyositis,” “polymyositis,” “anti-synthetase syndrome,” “systemic lupus erythematosus,” “Sjögren’s syndrome,” “vasculitis,” “interstitial pneumonia with autoimmune features,” “natural history” and “screening.” We selected the articles for this review based on their relevance to the natural history and the screening of interstitial lung disease in systemic autoimmune rheumatic disorders. We focused on recent publications and on studies with large series of patients. Manuscripts not written in English were excluded.
Pathogenesis and clinical aspects of SARDs-ILDs
Many different pathogenetic mechanisms are implicated in the development of ILD in the context of SARDs. The initial insult results in recruitment of inflammatory cells into the interstitial and alveolar spaces of the lung, causing alveolar epithelial damage. Consequently, fibroblasts and myofibroblasts in the interstitial spaces of the lung become activated and produce extracellular matrix proteins, leading to lung fibrosis.11 Both immune cells and soluble mediators participate in the pathogenesis of ILD in SARDs. Immune cells involved in lung inflammation and fibrosis are T cells, B cells, M1 (classically activated) and M2 (alternatively activated) macrophages, neutrophils and fibrocytes, while soluble mediators include transforming growth factor-β (TGF-β), platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2), colony stimulating factor-1 (CSF-1), IL-1β, IL-13, IL-17 and chemokines such as CCL2, CCL17, CCL18 and CXCL12.5,12–14 On the other hand, environmental factors such as air pollution may contribute to the initial insult leading to pulmonary inflammation and fibrosis.15,16 Deregulated tissue remodeling is another major pathogenetic mechanism of lung injury. Repair of damaged tissues normally consists of an inflammatory phase, a fibroblast migration phase and a tissue remodeling phase in which normal tissue architecture is restored.17 In pulmonary fibrosis excessive production of extracellular matrix components by fibroblasts and myofibroblasts, such as hyaluronan, fibronectin and collagens, results in permanently remodeling of lung tissue structures, causing thickening of alveolar and peri-bronchial walls.12 Classically activated M1 macrophages participate in the early inflammatory phase of lung injury, while alternatively activated M2 macrophages seem to play a central role in the deregulated tissue remodeling by recruiting and activating fibroblasts /myofibroblasts through TGF-β, PDGF and CCL18.18 In a recent study, single-cell RNA-sequencing was performed in lung tissue specimens of patients with SSc related ILD, to investigate gene expression of mesenchymal cells.19 It was found that SSc-ILD compared to healthy lungs, are characterized by significant expansion of a myofibroblast population representing the main profibrotic effector cells in these patients. In addition, a distinct population of alveolar macrophages with profibrotic properties was found to be expanded in lungs of patients with pulmonary fibrosis, either idiopathic or related to SARDs.20 The interplay among fibroblasts/myofibroblasts, resident tissue lung cells and inflammatory cells of innate and acquired immunity create a tight cellular net which drives the fibrotic process. These complex interactions are extremely dynamic and are mediated by a milieu of key molecules largely unknown. New biotechnologies and in-depth dissection at the cellular and molecular tissue level of SARDs-ILD are expected to shed light and reveal fundamental mechanisms for maintaining and perpetuating lung injury and destruction. In addition, the mechanisms linking systemic autoimmunity with lung involvement have been poorly investigated and it is anticipated to interpret how a systemic autoimmune response can be expanded and overwhelm the regulatory barriers of tracheobronchial tree, leading to chronic inflammation and tissue damage.
The clinical picture of SARDs-ILDs varies and depends mainly on the histologic ILD subtype and the underlying rheumatic disease. Patients may be completely asymptomatic, but may also present with exertional dyspnea and/or dry cough, while productive cough is very uncommon. Exertional dyspnea is often of insidious onset with progressive worsening, although in some cases it is characterized by acute onset and may be accompanied by low grade fever. Other less common clinical manifestations include chest pain and hemoptysis. In progressively severe and untreated cases, dyspnea at rest may occur along with cyanosis and clubbing of digits due to prolonged hypoxemia. Long-standing cases of ILD may eventually lead to secondary pulmonary hypertension and clinical manifestations of cor pulmonale. Pleurisy may also accompany ILD in specific SARDs. Extrapulmonary manifestations are characteristic of the underlying SARDs and may include fatigue, low grade fever, arthralgias, arthritis, myalgias, muscle weakness, rash, Raynaud’s phenomenon, pericarditis and sicca symptoms along with hematologic abnormalities and specific immunologic profile.
Physical examination may be normal or may reveal bibasilar inspiratory crackles or “velcro rales” on auscultation, while in cases of secondary pulmonary hypertension increased intensity of the second heart sound with narrow splitting may occur. ILD diagnosis is based on HRCT or lung biopsy, while pulmonary function tests (PFTs) and arterial blood gases (ABGs) are used to evaluate the extent of the disease and the impairment level of pulmonary function. Since SARDs may be accompanied by several complications such as infections or secondary pulmonary hypertension, the diagnostic work up may also include bronchoscopy with bronchoalveolar lavage studies, lung biopsy, heart ultrasound and right heart catheterization. Table 1 summarizes the main characteristics of ILD in different SARDs.
Table 1.
Main characteristics of ILD in SARDs.
| Prevalence of ILD in SARD patients | ILD pattern | Risk factors | Outcome | |
|---|---|---|---|---|
| SSc-ILD | 21–71%21–27 | NSIP > UIP28 | Diffuse cutaneous SSc, Scl-70 + , male sex, older age, thoracic lymphadenopathy21–27,29 | Most common cause of SSc-related death30
Median survival of 11.2 years31 |
| RA-ILD | 2.2–10%32–37 | UIP > NSIP > COP38,39 | Older age, male sex, smoking, RF, ACPA and RA disease activity40,41 | Median survival of 2.6-7.8 years 10-year mortality of 60.1%32,37,42,43 |
| IIM-ILD | 19–40%44–47 (69–100% in anti-synthetase syndrome)47–49 | NSIP > COP > DAD/AIP > UIP50–52 | Antisynthetase antibodies,47–49 anti-MDA-5 antibodies, CADM53,54 | 5-year mortality of 53.3%44 |
| pSS-ILD | 13–20%55–58 | NSIP > UIP > COP, LIP59,60 | Older age, male sex, smoking, disease duration, non-sicca onset of disease, ANA, RF, anti-Ro-52, increased CRP60–64 | 5-year mortality of 11-39%62,65,66 |
| SLE-ILD | 8–10%67,68 | NSIP > UIP, COP, DAD/AIP, LIP69,70 | Older age, male sex, late onset of SLE71 | 5-year mortality of 14.7%72 |
| AAV-ILD | 7–47%73–78 | UIP > NSIP74,75,78,79 | MPA, MPO-ANCA73–78 | 5-year mortality of 16.2%79 |
| IPAF | – | NSIP, UIP80–82 | – | Mean survival of 6.1 years83 |
AAV-ILD, ANCA-associated vasculitis-interstitial lung disease; ACPA, anti-citrullinated protein antibodies; AIP, acute interstitial pneumonia; ANA, antinuclear antibodies; CADM, clinically amyopathic dermatomyositis; COP, cryptogenic organizing pneumonia; CRP, C-reactive protein; DAD, diffuse alveolar damage; IIM-ILD, idiopathic inflammatory myopathies-interstitial lung disease; ILD, interstitial lung disease; IPAF, interstitial pneumonia with autoimmune features; LIP, lymphoid interstitial pneumonia; MPA, microscopic polyangiitis; MPO-ANCA, myeloperoxidase-antineutrophil cytoplasmic antibodies; NSIP, nonspecific interstitial pneumonia; RA, rheumatoid arthritis; RF, rheumatoid factor; SARD, systemic autoimmune rheumatic disorders; sLE, Systemic lupus erythematosus; UIP, usual interstitial pneumonia.
Systemic sclerosis (SSc)
The prevalence of ILD in SSc patients varies widely between 34% and 60%, basically due to different methodologies and criteria used for the diagnosis of lung involvement,22,23,24,27 while the incidence rate has been recently estimated at 1364 cases per 100,000 patient-years.84 ILD is more common in diffuse SSc (dcSSc) compared to limited SSc (lcSSc), with reported prevalences ranging from 40–71% and 21–-53%, respectively.21–27 In addition, ILD occurs more frequently in patients with positive anti-topoisomerase I (anti-Scl-70) antibody, but may also be present in other SSc specific antibodies.25,27 Thoracic lymphadenopathy is another strong predictor of ILD presentation in SSc patients and its presence is associated with greater extent of lung involvement.29,85–87 Laboratory and imaging evaluation of SSc-ILD is mainly based on PFTs and HRCT respectively. PFTs in SSc-ILD disclose a restrictive pattern characterized typically by reduced forced vital capacity (FVC), normal forced expiratory volume in the first second (FEV1) to FVC ratio and various degree of reduced diffusing capacity of lungs for CO (DLCO).88 Imaging with HRCT more frequently shows an NSIP pattern in approximately 83% of patients, followed by UIP in 17%.28 Confirmation of SSc-ILD with lung biopsy is rarely necessary, since the ILD pattern can be usually diagnosed by HRCT findings.
Natural history of ILD in SSc
The clinical course of SSc-ILD is characterized by remarkable variability. Some patients present with a slow decline of pulmonary function, while in other there is a rapid progression after diagnosis, as reflected in worsening measures of FVC and/or DLCO. A retrospective study of 254 patients with SSc-ILD who received various treatments identified seven distinct FVC trajectories: very low FVC on baseline with slow decline (5.5%), very low baseline FVC with improvement (13.8%), low baseline FVC with rapid decline (9.5%), low baseline FVC that remained stable (19.7%), low-normal baseline FVC with improvement (31.1%), normal baseline FVC with improvement (16.1%) and normal baseline FVC that remained stable (4.3%).89 Recently, a study of 826 patients with SSc-ILD from the EULAR Scleroderma Trials and Research (EUSTAR) cohort found that 27% of SSc-ILD patients presented ILD progression defined as a relative decline in FVC of ⩾ 5% after 12 months. A total of 535 SSc-ILD patients from this cohort had a mean follow-up of 5 years and although in each 12-month period, 23–27% of patients presented ILD progression, only a minority of them progressed in consecutive 12-months periods. Among patients with ILD progression, most of them presented slow deterioration of pulmonary function, with more periods of stability or improvement, while only 8% had rapid, continuing decline of pulmonary function. Male sex, higher modified Rodnan skin score and reflux/dysphagia symptoms were independent predictors of ILD progression.90 Furthermore, patients with SSc seem to display a more rapid progression of ILD during the first 3 to 5 years of disease.91 Among 695 patients with SSc in the EUSTAR cohort, 31% had FVC < 80% of predicted value and 65% had DLCO < 80% within 1 year after Raynaud’s phenomenon onset. Three years after Raynaud’s phenomenon onset, approximately one-third of patients had DLCO < 50% of predicted values.92 Seventy-seven patients with SSc-ILD in the placebo arm of the Scleroderma Lung Study showed an annual decline of 4.2% and 8.2% in FVC and DLCO, respectively. Interestingly, the rates of decline in FVC were higher in the group with severe fibrosis at baseline HRCT and more pronounced in patients with disease duration of less than 2 years.93 However, Hoffmann-Vold and colleagues27 found no correlation between ILD progression estimated by PFTs decline and the extent of lung fibrosis on HRCT. Wu and colleagues developed the SpO2 and Arthritis (SPAR) model after studying two prospective cohorts of patients with SSc and limited extent of ILD (<20% HRCT extent). ILD progression was defined as a relative decline in FVC of ⩾ 15% or decline in FVC ⩾ 10% at baseline combined with decline in DLCO ⩾ 15% after one year follow-up from ILD diagnosis. In this study, O2 desaturation (⩽94%) after 6 minute walk test and the presence of arthritis were independent predictors of ILD progression.94 The INBUILD trial introduced a new definition for PF-ILD. The investigators defined progression of ILD within 24 months as a relative decrease in FVC predicted greater than 10% from baseline or as a decrease in FVC between 5% and 10% combined with deterioration of respiratory symptoms or increased extent of fibrosis on HRCT or third, as a deterioration of respiratory symptoms combined with increased extent of fibrosis on HRCT. The INBUILD trial enrolled 663 patients with different types of ILD meeting those criteria for progression, including patients with SSc-ILD, RA-ILD and other SARDs-ILDs. The placebo arm presented significant reduction in FVC, demonstrating the ability of the above definition of PF-ILDs to detect ILD patients with progressive disease.95 In a cohort study of 171 patients with SSc-ILD, Guler and colleagues reinforced the concept that patients can be classified into three subgroups with distinct progression patterns based on FVC decline and survival rates: long-term survival (> 8 years after diagnosis), medium-term survival (4-8 years after diagnosis) and short-term survival (deceased within < 4 years after diagnosis). Patients with short-term survival had a higher annual rate of decline in FVC than those with medium-term survival and the latter subgroup had a higher rate of decline in FVC than those with long-term survival,31 findings which highlighted the association of rate of decline in FVC with mortality.
ILD is a major cause of mortality in patients with SSc. SSc-ILD along with pulmonary hypertension have replaced scleroderma renal crisis as the primary cause of SSc-related deaths in recent decades.96 In a study from the EUSTAR database including 5860 patients with SSc, pulmonary fibrosis was the most common cause of SSc-related death, accounting for 35% of total mortality cases.30 Furthermore, Guler and colleagues31 found that the median survival of patients with SSc-ILD was 11.2 years from the time of ILD diagnosis. In a Norwegian cohort of 815 SSc patients, among which 324 patients had SSc-ILD, the 5- and 10-year survival rates of SSc patients with lung fibrosis were 69% and 56% respectively, whereas the same rates for SSc patients without pulmonary fibrosis were 83% and 80%.27
Prediction models for progression and mortality of SSc-ILD have been developed using different clinical variables. Baseline FVC and DLCO levels have been used for the assessment of SSc-ILD severity and along with disease extent on HRCT, they are predictors of mortality.97 In 2008, Goh and colleagues proposed a simple staging system for SSc-ILD, integrating PFTs and HRCT. Patients with disease extent > 20% on HRCT were classified as having extensive disease, whereas patients with disease extent < 20% were classified as having limited disease. For patients with indeterminate HRCT extent (between 10% and 30%), the FVC threshold of 70% was used for classification into extensive or limited disease subgroup. Furthermore, it was shown that the extensive disease staging system was a powerful predictor of mortality with better performance than HRCT or FVC alone.97 Furthermore, Hoffmann-Vold and colleagues27 showed that mortality in patients with SSc-ILD correlated both with the extent of lung fibrosis on HRCT even in the absence of impaired lung function and with lower baseline FVC.
Serial measurements of pulmonary function are also predictors of mortality. In a study of 162 patients with SSc-ILDs, the prognostic significance of annual PFT changes was examined against 15-year survival. The optimal predictor of mortality was a relative FVC decrease of ⩾ 10% from baseline or an FVC decrease of 5-9% combined with a DLCO decrease of ⩾ 15% compared to baseline.98 Volkmann and colleagues used Cox proportional hazards models to identify predictors of survival in patients with SSc-ILD who participated in the Scleroderma Lung Studies I and II. It was found that decline in FVC ⩾ 10% and in DLCO ⩾ 15% compared to baseline over a 2 year-period were independent predictors of mortality, in addition to high baseline skin score and older age at randomization.99 Besides, in the Norwegian cohort of SSc patients mentioned previously, older age, male sex, higher Rodnan skin score, lower baseline FVC, baseline fibrosis, FVC decline and systolic pulmonary arterial pressure were associated with increased mortality.27 Furthermore, Morisset and colleagues100 examined two cohorts of SSc-ILD patients and developed an all-cause mortality prediction model that classifies patients into low, moderate and high three year mortality risk, according to smoking history, age and DLCO. Although, further validation of these prediction models is needed, they may be helpful for risk stratification of patients with SSc-ILD in clinical practice. Therefore, symptoms severity, patients reported outcomes focusing on quality of life and daily activities, along with a pulmonary assessment including radiographic pattern and extent, FVC and DLCO measurements, disease phenotype and demographic features should be evaluated in all patients with SSc, to estimate the likelihood of progression and mortality risk and design appropriate treatment intervention.
Screening of ILD in SSc
Because many patients with SSc who have ILD may be asymptomatic, it is clinically important to screen all SSc patients for ILD and diagnose ILD as early in the disease course as possible, in order to offer therapeutic interventions capable of changing the natural history of the disease. The main methods, routinely used for the evaluation of ILD involvement in SSc are HRCT, PFTs and ABGs. Although PFTs are a useful and noninvasive tool for ILD evaluation and disease progression, there are certain limitations of these tests in patients with SSc. Normal values of PFTs have a wide range (80-120%) and ILD may be present despite “normal” PFTs predicted values. Indeed, three studies have shown that PFTs lack sensitivity for ILD detection in SSc.101–103 In a prospective study of 102 patients with SSc, Suliman and colleagues found that among 64 patients with ILD on HRCT, only 37.5% had FVC values below 80% of predicted. Combining FVC < 80% and DLCO < 70% only increased sensitivity to 59%. A higher sensitivity level of 72% was achieved when the following parameters were combined: FVC < 80% of predicted or ΔFVC > 10% or TLC < 80% or DLCO < 70% and FEV1/FVC over 0.7. However, specificity was as low as 42%.103 In another prospective study of 305 patients with SSc, Hoffman-Vold and colleagues101 found a similar sensitivity level for FVC < 80% at baseline screening of patients with SSc-ILD. In a similar study of 265 patients with SSc, Showalter and colleagues identified FVC < 80% (sensitivity 69%, specificity 73%) and DLCO < 62% (sensitivity 60%, specificity 70%) as the optimal thresholds for SSc-ILD detection. However, all FVC and DLCO combinations had negative predictive values lower than 0.7.102
HRCT is the gold standard for diagnosis of ILD and allows evaluation of the radiologic pattern and the extent of the disease. Launay and colleagues104 showed the value of baseline HRCT in a study of 90 patients with SSc, in which 34 of 40 (85%) patients with normal baseline HRCT still had normal imaging after 5-year follow-up. Furthermore, in a prospective study of 305 patients with SSc, Hoffmann-Vold and colleagues101 found that 108 patients with no lung fibrosis on HRCT at baseline remained disease free after a mean follow-up of 3.1 years.
Lately, novel methods have been evaluated for ILD detection in patients with SSc, including lung ultrasound, lung MRI and specific serum biomarkers. A recent study aimed to assess the value of lung ultrasound as a screening tool for SSc-ILD compared to HRCT. Hassan and colleagues performed lung ultrasound to examine B-lines in 67 patients with SSc, 29 of whom had ILD on HRCT. Lung ultrasound had 100% sensitivity but it was accompanied by very low specificity of 34%.105 Fairchild and colleagues chose to evaluate pleural changes (pleural irregularity, thickening and granularity) instead of B-lines with lung ultrasound. After evaluating 20 patients with SSc, 9 of whom had ILD on HRCT, pleural changes detected by lung ultrasound had 100% sensitivity and 82% specificity for the detection of ILD.106 Although promising, lung ultrasound requires validation in future studies before being introduced in every-day clinical practice.107 In addition, an exploratory study evaluated two-breath hold, inspiratory and expiratory MRI of the lungs for the detection of lung fibrosis in 16 patients with SSc and revealed that MRI had 86% sensitivity and 75% specificity compared to HRCT.108 Finally, serum KL-6, SP-D and CCL18 levels have been tested and shown to be useful biomarkers for detecting SSc-ILD, but further studies are required to confirm their clinical value.109
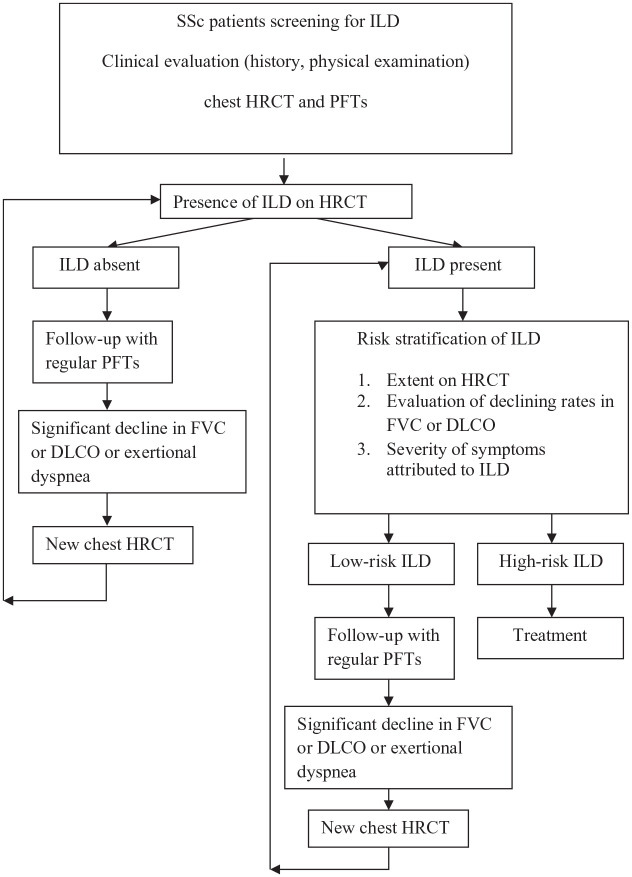
There are to date no guidelines for ILD screening in SSc, but an evidence-based consensus statement on identification and management of SSc-ILD was published recently.110 They proposed that all patients with SSc should be screened for ILD with HRCT, PFTs at baseline and auscultation, contributing to assessment of severity and prognosis. They also proposed that PFTs should be repeated regularly and deterioration of PFTs or appearance of new symptoms should warrant evaluation with a follow-up HRCT. Physical performance tests, such as six-minute-walk test and exercise-induced oxygen desaturation may also contribute to the assessment of ILD severity.110 Figure 1 depicts a proposed algorithm for screening and assessment of SSc-associated ILD.
Figure 1.
Proposed algorithm for screening and assessment of SSc-ILD patients based on risk factors associated with increased mortality.27,31,97,99 High-risk patients: one or more of the following risk factors including ILD extent on HRCT, low baseline FVC and/or DLCO, decline in FVC ⩾ 10%, decline in DLCO ⩾ 15%, higher modified Rodnan skin score. Low-risk patients: absence of any risk factor.
Rheumatoid arthritis (RA)
ILD is common among patients with RA, but estimates of its prevalence vary depending on methodology and whether clinically apparent or subclinical forms are investigated and published. The prevalence of clinically significant ILD in RA ranges between 2.2% and 10%32–37 while estimates of subclinical ILD are as high as 58%.111 The incidence rate of ILD in RA patients has been estimated at 109 cases per 100,000 patient-years.84 Risk factors associated with ILD development in RA in many, but not all studies include older age at disease onset, male sex, smoking, seropositivity for rheumatoid factor (RF) and anticitrullinated protein antibodies (ACPAs) as well as high RA disease activity.40,41 In contrast to other autoimmune rheumatic disorders where NSIP is the most frequent ILD pattern, UIP predominates in RA, affecting approximately 50% of ILD patients, followed by NSIP and COP, whereby a predominance of NSIP has been reported in patients from China.38,39
Natural history of ILD in RA
The clinical course of ILD in RA is quite heterogeneous, with some patients remaining relatively stable or following a slow progression while others display a rapid deterioration of pulmonary function.112 Patients with RA-ILD and UIP pattern on HRCT seem to have a worse clinical course than those with non-UIP pattern.112,113 UIP is associated with more hospitalizations due to respiratory causes, more frequent use of oxygen therapy and more rapid decline of DLCO.112 Zamora-Legoff and colleagues conducted a retrospective study of 167 patients with RA-ILD in order to identify predictors for disease progression. It was found that UIP pattern, lower baseline DLCO and FVC values, and higher rates of PFTs decline within the first 6 months were associated with increased risk of RA-ILD progression, defined as a decline of DLCO or FVC < 50% or 40% of predicted values, respectively.113
The clinical course of RA-ILD may be complicated by acute exacerbations, initially described in idiopathic pulmonary fibrosis (IPF) but also reported in ILD related to systemic autoimmune rheumatic disorders.114 Acute exacerbations are characterized by rapid worsening of respiratory symptoms, hypoxemia, and new bilateral ground-glass opacities or consolidations on HRCT, in the absence of other causes such as infection or heart failure.114 Risk factors for acute exacerbations among patients with RA-ILD include older age at ILD diagnosis, UIP pattern on HRCT and methotrexate treatment. In addition, acute exacerbations in patients with RA-ILD are a poor prognostic factor, since they are associated with 2.47-fold increased mortality.115
ILD is a serious complication of RA and is accompanied by markedly increased mortality. Observational studies report a median survival of 2.6-7.8 years for patients with RA-ILD and 3-times higher risk of death than patients with RA who do not have ILD.32,37,42,43 A Danish population-based cohort study including 679 patients with RA-ILD and 11,722 patients with RA but no ILD showed that one-year mortality in RA-ILD was 13.9% compared to 3.8% in those without ILD, while 10-year mortality was 60.1% and 34.5%, respectively.37 In a retrospective study of 82 patients with RA-ILD, Kim and colleagues found that patients with UIP had worse survival than patients with non-UIP RA-ILD, with median survival of 3.2 years compared to 6.6 years in the latter group. Interestingly, survival of patients with UIP pattern RA-ILD was similar to that of patients with IPF.116 A recent meta-analysis of 1256 patients with RA-ILD reported that UIP pattern was associated with 1.6-fold higher mortality compared to other patterns.117
Aside of the HRCT pattern of ILD, pulmonary function is also a strong predictor of mortality in RA-ILD. In a study of 137 patients with RA-ILD, Solomon and colleagues found that patients with UIP had worse survival and higher declining rates of pulmonary function than those with NSIP. Furthermore, low baseline FVC and 10% decline in FVC from baseline at any time during follow-up were identified as predictors of mortality.118 However, multivariate analysis, after adjusting for age, sex, smoking, baseline FVC and changes of FVC over time, did not demonstrate HRCT pattern as an independent predictor of mortality as opposed to worse pulmonary function at baseline and significant decline in pulmonary function over time.118 In another study of 181 patients with RA-ILD, Zamora-Legoff and colleagues119 showed that older age, longer RA disease duration and low baseline DLCO, but not HRCT pattern, were identified as independent predictors of mortality. Finally, Morisset and colleagues120 after analyzing a multi-center cohort of 309 patients with RA-ILD, pointed out that the GAP (gender, age, physiology) model was useful as a predictor of mortality, similarly to IPF.
Screening of ILD in RA
The major impact on mortality of ILD in patients with RA and the often asymptomatic pulmonary disease course excludes screening strategies relying solely on clinical signs and the presence of respiratory symptoms for RA-ILD detection. However, the aforementioned risk factors for RA-ILD development (older age, male sex, smoking, positive RF or ACPAs, high RA disease activity) may be useful for identifying high-risk patients, in whom physical examination, baseline PFTs and HRCT could be used for detection and monitoring of ILD. In patients with ILD, PFTs should be repeated regularly while deterioration of PFTs or occurrence of new symptoms should warrant further evaluation with new HRCT. However, there are no studies proposing and validating a particular screening algorithm for RA-ILD.
Serum biomarkers that could be used for identification of high-risk patients and early diagnosis of RA-ILD have been investigated by several groups. Doyle and colleagues studied risk factors associated with ILD in two cohorts of 113 and 76 patients with RA. Age, sex, smoking history, positive RF and ACPA, along with a combinatorial signature of serum biomarkers including matrix metalloproteinase 7 (MMP7), pulmonary and activation-regulated chemokine (PARC) and surfactant protein D (SP-D) were investigated as possible risk factors for RA-ILD. The first cohort of 113 patients with RA was used to construct the logistic regression derived formula based on the risk factors incorporated into the model. Subsequently, the tool was validated in the second cohort of 76 patients with RA, exhibiting 87% sensitivity and 92% specificity for RA-ILD diagnosis.121 A further study of 620 patients with RA-ILD, 614 RA patients without ILD and 5448 unaffected controls enrolled in a large case-control study demonstrated that the MUC5B promoter variant rs35705950 was associated with a three-fold increased risk of ILD among patients with RA. However, this particular polymorphism was specifically associated with the UIP pattern.122 Other potential biomarkers for detection and screening of RA-ILD include serum KL-6 protein,123,124 anti-malondialdehyde-acetaldehyde antibodies,125 tumor markers CA15-3, CA125 and CA19-9.126 However, to date, none of these are validated and used in clinical practice.
Recently, lung ultrasound has been assessed for the detection of ILD in patients with RA. Mozaedi-Fuerst and colleagues evaluated the presence of B-lines and pleural nodules with lung ultrasound in 64 patients with RA who had no respiratory symptoms. HRCT was obtained in these patients and used as the gold standard for underlying ILD diagnosis. Ultrasound was found to have 97.1% sensitivity and 97.3% specificity for the detection of subclinical ILD.127 Furthermore, Manfredi and colleagues128 recently developed an algorithm to detect velcro crackles in recorded pulmonary sounds from an electronic stethoscope in order to screen patients with RA for lung disease. The algorithm was tested in 137 patients with RA, and sensitivity was 93.2% accompanied by 76.9% specificity for ILD detection confirmed by HRCT.128 Larger studies are needed to validate and confirm the clinical utility of the recently proposed screening and diagnostic tools for RA-ILD.
Idiopathic inflammatory myopathies (IIM)
Dermatomyositis (DM) and polymyositis (PM) are typically classified into idiopathic inflammatory myopathies (IIM), with ILD being the most common extra-muscular manifestation, ranging from 19% to 40%.44–47 In a prospective cohort of 23 patients with DM/PM who underwent repeated investigation with HRCT and PFTs irrespective of clinical manifestations, the prevalence of both subclinical and clinical ILD reached 78%.129 The incidence rate of ILD in DM patients has been estimated at 1011 cases per 100,000 patient-years and in PM patients at 831 per 100,000 patient-years.84 The most common histopathological ILD pattern in patients with myositis is NSIP, followed by COP, DAD/AIP and UIP.50–52 Patients with IIM and ILD present higher extramuscular disease activity and are characterized by more severe disease damage.47
A subgroup of patients with DM/PM has anti-synthetase syndrome, which is characterized by the presence of anti-aminoacyl-tRNA synthetase antibodies (including anti-Jo-1, anti-Ej, anti-Oj, anti-PL7, anti-PL12), ILD and several clinical manifestations, including fever, arthralgia, Raynaud’s phenomenon and mechanic’s hands. The prevalence of ILD in anti-synthetase syndrome ranges between 69% and 100% and almost all myositis patients with anti-synthetase antibodies will eventually develop ILD.47–49,130 Notably, anti-PL7 and anti-PL12 antibodies have been associated with more severe lung involvement and lower baseline FVC and DLCO in patients with anti-synthetase syndrome compared to anti-Jo-1 antibodies.131 Another subset of DM patients develop anti-MDA-5 antibodies against RNA helicase which is encoded by melanoma-differentiation-associated gene 5. Patients with anti-MDA-5 antibodies share similar clinical features with anti-synthetase syndrome, such as ILD, fever, inflammatory arthropathy and mechanic’s hands. However, patients with detected MDA-5 may also have cutaneous manifestations, including cutaneous ulcerations and tender palmar papules. Approximately half of anti-MDA-5 positive patients have no muscle weakness, constituting the so-called DM variant of clinically amyopathic dermatomyositis (CADM).53,54 The prevalence of ILD in patients with anti-MDA-5 antibodies is high, ranging from 72% to 100%.53,54 It is noteworthy that these patients may present a rapidly progressive form of ILD which responds poorly to treatment and may lead to end-stage respiratory failure.53
Natural history of ILD in IIM
In patients with DM/PM and ILD, muscle and lung involvement may present concurrently, but ILD may also precede or follow myositis. ILD tends to occur early during the myositis course and usually dominates the clinical picture.132 The clinical course of ILD in DM/PM follows three different patterns: progressive form with acute onset, chronic form with slow progression and asymptomatic form.132 In a series of 107 patients with DM/PM related ILD, 18.7% presented with acute onset ILD, 51.4% with progressive lung manifestations, and 29.9% were asymptomatic. After treatment, 15.9% of patients had continued deterioration in pulmonary function as defined by decline of ⩾ 10% in FVC or ⩾ 15% in DLCO over a 34 month-follow-up period.132 Factors associated with ILD progression were older age at ILD onset, symptomatic ILD, low FVC and DLCO at baseline and a UIP pattern.132 In another series of 75 patients with anti-synthetase syndrome and ILD under treatment, 8% had deterioration in FVC ⩾ 10% or in DLCO ⩾ 15% after 1 year of follow-up.133
IIM related ILD may present with a rapidly progressive course, characterized by acute onset of respiratory symptoms, fever and/or malaise along with significant deterioration of pulmonary function and hypoxemia over a period of weeks. Rapidly progressive ILD usually has an HRCT pattern of acute interstitial pneumonia (AIP) with histopathological findings of diffuse alveolar damage (DAD).134 It is often associated with clinically amyopathic dermatomyositis (CADM) and anti-MDA-5 antibodies. Rapidly progressive ILD in myositis is associated with high mortality rates leading to fatal respiratory failure, and therefore intensive immunosuppression treatment should be introduced as soon as possible after diagnosis.53
ILD confers increased mortality risk among patients with DM/PM. In a series of 30 patients with clinically apparent myositis-related ILD, the 5 year-mortality was estimated to 53.3%. Poor survival was associated with male sex and presentation of AIP.44 In another series of 114 patients with myositis-related ILD, mortality was found to be 27.2%. Predictors of mortality in this series were acute form of ILD, CADM, older age and lower baseline FVC. Patients with the acute form of ILD had a significantly lower 5-year survival rate compared to those with the chronic form (52% vs 87% respectively), while CADM-ILD had worse 5-year survival than DM-ILD and PM-ILD (59% vs 71% vs 82% respectively).52 Another study of 107 patients with DM/PM associated ILD demonstrated that progression of ILD with decline of FVC and DLCO was a strong predictor of mortality.132 Furthermore, male sex, older age, lower baseline FVC and DLCO, presentation with acute/subacute interstitial pneumonia and extent of radiological abnormality have been linked to increased mortality in patients with DM/PM related ILD.135,136
Screening of ILD in IIM
Considering the high prevalence of ILD in patients with myositis and the increased morbidity and mortality of this particular complication, all patients with IIM, and especially those with anti-synthetase syndrome or anti-MDA-5 antibodies, should be screened with HRCT and PFTs as the initial baseline evaluation. In patients with ILD, PFTs should be repeated regularly to monitor disease progression while deterioration of PFTs or new onset respiratory symptomatology should warrant evaluation with new HRCT. Especially in IIM cases of AIP or ILD of acute onset, an underlying opportunistic or community acquired infection should be excluded by urgent bronchoscopy and bronchoalveolar lavage studies before administrating intensive immunosuppressive treatment.
Sjögren’s syndrome
Lung involvement in primary Sjögren’s syndrome (pSS) includes interstitial lung disease, small airway disease, xerotrachea and lymphoproliferative disorders.137 Estimates of prevalence of ILD in patients with pSS ranges from 13% to 20% in the majority of studies.55–58 Interestingly, prevalence of ILD in pSS seems to be associated with disease duration: the cumulative prevalence of ILD in patients with pSS was 10% after 1 year from pSS diagnosis and increased to 20% at 5 years and 43% at 15 years following pSS diagnosis.57 The incidence rate of ILD in pSS patients has been estimated at 196 cases per 100,000 patient-years.84 Risk factors associated with the development of ILD in these patients include male sex, older age, smoking, long pSS disease duration, non-sicca disease onset, positive anti-nuclear antibodies, rheumatoid factor and anti-Ro-52 autoantibodies and increased C-reactive protein (CRP).60–64 The most frequent HRCT pattern of ILD in pSS is NSIP (41–45%), followed by UIP (10%), COP (4%) and LIP (4–8%). A combination of different imaging patterns has been also observed.59,60
Natural history of ILD in pSS
The time of ILD occurrence in pSS varies. It may present late in the course of pSS, simultaneously with other manifestations of the disease or it may precede the onset of clinically apparent pSS.56,57 In a series of 21 patients with pSS-ILD, Roca and colleagues showed that the onset of ILD may be acute/subacute, symptomatic or asymptomatic. In this study, 15.8% of patients were improved after immunosuppressive treatment, 47.4% remained stable while 36.8% deteriorated as reflected in a decline in FVC ⩾ 10% or in DLCO ⩾ 15% after two years of follow-up.138 In another series of 18 patients with pSS-ILD, 28% of patients had deterioration of pulmonary function after 38 months of median follow-up.66 A more recent series of 49 patients with pSS-ILD in whom repeat PFTs were obtained six months from baseline found that 20.4% of patients progressed with decline of lung function with UIP pattern being a risk factor for progression.139 Furthermore, patients with pSS-ILD may rarely present with an acute exacerbation of ILD characterized by rapid deterioration of respiratory function and symptoms, resulting in high mortality.140 Data on prognosis and mortality of ILD in pSS are varying. Five-year survival rates in two series of patients with pSS-ILD, one with 165 and another with 33 patients, ranged between 84% and 89%.62,65 In another series of 18 patients with pSS-ILDs, 39% died after a median follow-up of 38 months, including three deaths from acute exacerbation of ILD.66 Risk factors associated with mortality are a higher extent of reticular abnormality on HRCT, honeycombing, higher partial pressure of carbon dioxide in arterial blood (PaCO2), lower partial pressure of oxygen in arterial blood (PaO2) and extent of fibroblastic foci on biopsy.65,141
Screening of ILD in pSS
There are no studies suggesting a screening algorithm for ILD in patients with pSS. Considering that the prevalence of ILD in pSS is lower than that of SSc or IIM and that the clinical course of pSS-ILD is quite heterogeneous with progressive or stable course, identification of risk factors for lung involvement in pSS is a clinical unmet need. Pulmonary assessment of these patients is complicated by clinical features of the disease itself, including dry cough and dyspnea resulting from xerotrachea, bronchitis and bronchiolitis, which may mimic ILD. In these cases, extensive work up may be necessary focusing on HRCT, PFTs before and after bronchodilation and additional tests such as ABGs and six-minute walk time.
Systemic lupus erythematosus (SLE)
Interstitial lung disease is rare in SLE. The prevalence of clinically apparent ILD in SLE patients has been reported from 8% to 10%,67,68 while the incidence rate has been estimated at 120 per 100,000 patient-years.84 NSIP is the most frequent pattern on HRCT, but UIP, COP, DAD, and LIP may also occur.69,70 SLE-ILD more frequently affects older patients, men and patients with late onset SLE. It may present acutely as the result of acute lupus pneumonitis (ALP) or follow a more insidious course with exertional dyspnea and dry cough.71 ALP is a serious manifestation of SLE characterized by the acute onset of fever, dyspnea, cough and hypoxemia. The prevalence of ALP in patients with SLE is estimated 1–4%, and it has a high mortality rate reaching up to 50%. Imaging with HRCT demonstrates bilateral ground-glass opacities and consolidations. In a series of 55 patients with SLE-ILD, chronic ILD was more frequent (63.6%), followed by subacute (20%) and acute form of ILD (12.7%). The 5-year survival rate was 85.3%, while smoking, thrombocytopenia, neuropsychiatric manifestations, anti-ds-DNA titers, mixed NSIP and COP pattern and high extent of fibrosis on HRCT were prognostic factors associated with increased mortality.72 Prompt diagnosis is necessary in order to begin appropriate immunosuppressive treatment, but ALP may be challenging to differentiate from severe infections or acute respiratory distress syndrome (ARDS). Patients who survive may still present ILD as a sequelae of AIP.142,143 No screening strategy is recommended for ILD in SLE. Diagnostic modalities such as HRCT and PFTs mentioned previously should be employed according to clinical judgment.
ANCA-associated vasculitis (AAV)
ANCA-associated vasculitis including granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA) may be complicated by three distinct types of lung involvement: diffuse alveolar hemorrhage (DAH), lung nodules and ILD. DAH is attributed to capillaritis, which presents acutely with dyspnea, cough and hemoptysis, and may be part of any AAV subtype. Lung nodules usually represent granulomas, which are mostly encountered in GPA, are asymptomatic or incidentally discovered on HRCT. ILD is more common in MPA compared to the other forms of AAV.77 The prevalence of ILD in MPA varies significantly between 7% and 47%, with patients of Asian descent displaying the highest frequency, while the prevalence in GPA ranges from 9% to 17%.73–78 However, in a Japanese series of 156 patients with AAV, 2 of 14 patients with EGPA had ILD.77 UIP is the most common imaging pattern on HRCT, followed by NSIP.74,75,78,79,144,145
ILD occurs concurrently with the other manifestations of AAV, but it may also appear late in disease course or may rarely precede the other manifestations.74,75,79,144 Interestingly, patients with idiopathic interstitial pneumonias (IIP) and IPF with positive MPO-ANCA are at high risk for eventually developing overt MPA. In a Japanese series of 504 patients with IPF, 4% had MPO-ANCA and 3.2% had PR3-ANCA. During a median follow-up time of 5 years, an additional 5.7% and 5.3% developed MPO-ANCA and PR3-ANCA respectively. Nine (25.7%) of 35 patients with positive MPO-ANCA developed MPA after 5 years, but none of the patients positive for PR3-ANCA developed clinical AAV.146 Similarly, in a series of 305 patients with IIP, 26 (8.5%) had MPO-ANCA, with 9 (3%) patients developing MPA after 5 years.147
ILD is a serious and progressive complication of AAV, leading to increased mortality. In a series of 24 patients with AAV and ILD, it was found that pulmonary function was progressing over time.145 In addition, ILD presence among patients with MPA appeared to decrease life expectancy by almost 50%148 and was associated with a 4-fold higher mortality.149 In a series of 62 French patients with AAV and ILD, the 1-, 3- and 5-year survival rates were 93.5%, 89.6% and 83.8% respectively, with older age at AAV diagnosis, alveolar hemorrhage and UIP pattern being associated with increased mortality.79
Since lung involvement is a very common target of AAV, patients with AAV should undergo pulmonary assessment, although no recommendations have been formulated. Patients with AAV in clinical remission, and especially those suffering from MPA, who present with progressively worsening dyspnea or dry cough, should be further evaluated with HRCT and PFTs to evaluate for the presence of an ongoing inflammatory process leading to ILD.
Interstitial pneumonia with autoimmune features (IPAF)
Distinguishing ILD associated with SARDs from IIP, especially IPF, may be challenging but has clinical importance, as SARD-ILDs have better prognosis and their treatment is different.150 Many patients diagnosed with IIP have clinical features suggestive of an underlying systemic autoimmune disease but they are not diagnosed with a particular SARD. In 2015, the European Respiratory Society and American Thoracic Society formed a task force that suggested the term “Interstitial Pneumonia with Autoimmune Features” (IPAF) in order to describe these patients and proposed specific classification criteria (Table 2).3 This term is not validated for clinical use and is to date often used for research purposes.
Table 2.
Classification criteria for IPAF.
| 1. Presence of ILD (by HRCT or lung biopsy) and 2. Exclusion of other causes and 3. Does not fulfill criteria of a specific CTD and 4. At least one feature from at least two of these domains: A. Clinical domain B. Serologic domain C. Morphologic domain |
| A. Clinical domain 1. Mechanic’s hands 2. Digital tip ulcers 3. Inflammatory arthritis or polyarticular morning joint stiffness ⩾60 min 4. Palmar telangiectasia 5. Raynaud’s phenomenon 6. Digital oedema 7. Gottron’s sign |
| B. Serologic domain 1. ANA ⩾1:320, diffuse, speckled, homogeneous patterns or a. ANA nucleolar pattern (any titer) or b. ANA centromere pattern (any titer) 2. Rheumatoid factor ⩾2× upper limit of normal 3. Anti-CCP 4. Anti-dsDNA 5. Anti-Ro (SS-A) 6. Anti-La (SS-B) 7. Anti-ribonucleoprotein 8. Anti-Smith 9. Anti-topoisomerase (Scl-70) 10. Anti-tRNA synthetase 11. Anti-PM-Scl 12. Anti-MDA-5 |
| C. Morphologic domain 1. Radiologic patterns: a. NSIP b. OP c. NSIP with OP overlap d. LIP 2. Histopathology patterns or features: a. NSIP b. OP c. NSIP with OP overlap d. LIP e. Interstitial lymphoid aggregates with germinal centers f. Diffuse lymphoplasmacytic infiltration (with or without lymphoid follicles) 3. Multi-compartment involvement (in addition to ILD): a. Unexplained pleural effusion or thickening b. Unexplained pericardial effusion or thickening c. Unexplained intrinsic airways disease (by PFT, HRCT or biopsy) d. Unexplained pulmonary vasculopathy |
ANA, antinuclear antibodies; anti-SS-A, anti-Sjögren’s syndrome-related antigen A autoantibodies; anti-SS-B, anti-Sjögren’s syndrome-related antigen B autoantibodies; CTD, connective tissue disease; HRCT, high resolution computed tomography; ILD, interstitial lung disease; LIP, lymphoid interstitial pneumonia; NSIP, nonspecific interstitial pneumonia; OP, organizing pneumonia; PFT, pulmonary function tests.
After publication of the proposed criteria by the ERS-ATS task force, a number of retrospective and prospective observational studies have included cohorts of patients with IPAF. The most prominent clinical feature described by these cohorts is Raynaud’s phenomenon and the most frequent serologic finding ANA positivity.80–82 Most cohorts report NSIP as the most common pattern on HRCT.80,81,151,152 Patients with IPAF appear to have overall better survival than those with IPF (mean survival 73.3 vs 52.0 months respectively), but worse compared to CTD-ILD (mean survival 104 months).83 Furthermore, Oldham and colleagues82 have shown that age at diagnosis and lower baseline DLCO were independent predictors of mortality in patients with IPAF. Sebastiani and colleagues found that apart from DLCO at baseline, FVC was also an independent risk factor of increased mortality. Approximately 35% of patients with IPAF had ⩾ 10% decline in FVC and ⩾ 15% decline in DLCO after 12 months of follow-up.153 Age at disease diagnosis, baseline FVC, ILD exacerbations and ILD subtype were also demonstrated as risk factors of mortality.83
Recently, two interesting studies investigated different serum and BAL biomarkers in IPAF. Wang and colleagues showed that serum SP-A and KL-6 levels are increased in patients with IPAF, and that these levels are negatively correlated with FVC and DLCO. In addition, higher levels of SP-A and KL-6 predicted severe decline in pulmonary function.154 In addition, Kameda and colleagues155 demonstrated that serum and BAL levels of CXCL9, CXCL10 and CXCL11 were significantly increased in patients with IPAF and CTD-ILD compared to IPF. Further studies are needed to validate the clinical significance of these novel biomarkers before becoming part of routine clinical assessment of patients with IPAF.
Conclusion
ILD has been recognized as a frequent manifestation of several SARDs and most commonly presents with NSIP or UIP imaging pattern. In many patients with various forms of SARDs, ILD complication may be the most influential disease feature determining the severity and clinical course and outcome of the underlying disease, either as direct involvement of lung parenchyma or indirectly as a consequence of related co-morbidities such as infections and secondary pulmonary hypertension.
Both clinically apparent and limited extent of ILD impact mortality. The ongoing inflammation within the interstitium of lung tissues, coupled with the deregulated remodeling process, progressively leads to extensive fibrosis with irreversible and permanent tissue damage. The loss of normal architecture and the subsequent functional abnormalities eventually interfere with gas exchange, leading to hypoxemia and respiratory failure. Emerging data provide insights into ILD pathogenesis. It seems that independently of the initial cause and the underlying mechanisms, fibrosis is the “bottom neck” of ILD and specific cell subpopulations seem to mediate this process. In addition, key molecules ensuring the interlinking among different cell type have not been identified yet. Current treatment modalities are expected to work on two levels: anti-fibrotic agents blocking the fibrosis cascade and immunosuppression aiming to control chronic inflammation. Assuming that inflammation is coupled to fibrotic process, novel treatments may serve by blocking this interaction leading to the breakdown of this positive feedback loop.
The fact that many SARD-ILD cases follow an insidious and slowly progressive course highlights the importance of identifying the underlying ILD condition as soon as possible. Clinical course and management differ by underlying SARD and the nature of the lung involvement. Thus, from a clinical standpoint, early detection and diagnosis of ILD offers the opportunity to suppress the long-standing inflammation within lung interstitium, restrict the subsequent fibrotic response and limit the irreversible tissue damage. In the era of targeted treatments, it is important to efficiently screen patients with SARDs to detect ILD early and modify the otherwise adverse natural history of the disease. In the field of SARDs-ILD, precision medicine is anticipated to utilize novel biomarkers for early diagnosis, classification, monitoring, prognosis and above all tailoring targeted therapies based on specific phenotypic, cellular or molecular key characteristics.
Footnotes
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: E.M.: Consultant/advisory board, speaker (Boehringer-Ingelheim); speaker: (Novartis); editor, writer (UpToDate). A.M.H.V. has received research funding and/or consulting fees and/or other remuneration from Actelion, Boehringer Ingelheim, Roche, Bayer, Merck Sharp & Dohme, ARXX, Lilly and Medscape. P.P., A.G. and A.T. declare no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Panagiotis Panagopoulos  https://orcid.org/0000-0002-1630-7045
https://orcid.org/0000-0002-1630-7045
Anna Maria Hoffmann-Vold  https://orcid.org/0000-0001-6467-7422
https://orcid.org/0000-0001-6467-7422
Contributor Information
Panagiotis Panagopoulos, Department of Pathophysiology, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Andreas Goules, Department of Pathophysiology, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Anna Maria Hoffmann-Vold, Department of Rheumatology, Oslo University Hospital, Oslo, Norway.
Eric L. Matteson, Division of Rheumatology, Mayo Clinic College of Medicine and Science, Rochester, MN, USA
Athanasios Tzioufas, Department of Pathophysiology, School of Medicine, National and Kapodistrian University of Athens, Mikras Asias 75, Athens 11527, Greece.
References
- 1.Fischer A, du Bois R.Interstitial lung disease in connective tissue disorders. Lancet 2012; 380: 689–698. [DOI] [PubMed] [Google Scholar]
- 2.Karakontaki FV, Panselinas ES, Polychronopoulos VS, et al. Targeted therapies in interstitial lung disease secondary to systemic autoimmune rheumatic disease. Autoimmun Rev 2021; 20: 102742. [DOI] [PubMed] [Google Scholar]
- 3.Fischer A, Antoniou KM, Brown KK, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 2015; 46: 976–987. [DOI] [PubMed] [Google Scholar]
- 4.Kamiya H, Panlaqui OM.Systematic review and meta-analysis of the prognosis and prognostic factors of interstitial pneumonia with autoimmune features. BMJ Open 2019; 9: e031444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spagnolo P, Distler O, Ryerson CJ, et al. Mechanisms of progressive fibrosis in connective tissue disease (CTD)-associated interstitial lung diseases (ILDs). Ann Rheum Dis 2021; 80: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells AU, Brown KK, Flaherty KR, et al. What’s in a name? That which we call IPF, by any other name would act the same. Eur Respir J 2018; 51: 1800692. [DOI] [PubMed] [Google Scholar]
- 7.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez FJ, Collard HR, Pardo A, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers 2017; 3: 17074. [DOI] [PubMed] [Google Scholar]
- 9.Travis WD, Hunninghake G, King TE, Jr, et al. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am J Respir Crit Care Med 2008; 177: 1338–1347. [DOI] [PubMed] [Google Scholar]
- 10.Rubio-Rivas M, Royo C, Simeón CP, et al. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum 2014; 44: 208–219. [DOI] [PubMed] [Google Scholar]
- 11.Wells AU, Denton CP.Interstitial lung disease in connective tissue disease–mechanisms and management. Nat Rev Rheumatol 2014; 10: 728–739. [DOI] [PubMed] [Google Scholar]
- 12.Kolahian S, Fernandez IE, Eickelberg O, et al. Immune mechanisms in pulmonary fibrosis. Am J Respir Cell Mol Biol 2016; 55: 309–322. [DOI] [PubMed] [Google Scholar]
- 13.Rangel-Moreno J, Hartson L, Navarro C, et al. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest 2006; 116: 3183–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann-Vold AM, Weigt SS, Saggar R, et al. Endotype-phenotyping may predict a treatment response in progressive fibrosing interstitial lung disease. Ebiomedicine 2019; 50: 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H-H, Yong Y-M, Lin C-H, et al. Air pollutants and development of interstitial lung disease in patients with connective tissue disease: a population-based case–control study in Taiwan. BMJ Open 2020; 10: e041405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sack C, Vedal S, Sheppard L, et al. Air pollution and subclinical interstitial lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA) air–lung study. Eur Respir J 2017; 50: 1700559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynn TA.Integrating mechanisms of pulmonary fibrosis. J Exp Med 2011; 208: 1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Wang Y, Wu G, et al. Macrophages: friend or foe in idiopathic pulmonary fibrosis? Respir Res 2018; 19: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valenzi E, Bulik M, Tabib T, et al. Single-cell analysis reveals fibroblast heterogeneity and myofibroblasts in systemic sclerosis-associated interstitial lung disease. Ann Rheum Dis 2019; 78: 1379–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reyfman PA, Walter JM, Joshi N, et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med 2019; 199: 1517–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diab S, Dostrovsky N, Hudson M, et al. Systemic sclerosis sine scleroderma: a multicenter study of 1417 subjects. J Rheumatol 2014; 41: 2179–2185. [DOI] [PubMed] [Google Scholar]
- 22.Dougherty DH, Kwakkenbos L, Carrier ME, et al. The Scleroderma Patient-Centered Intervention Network Cohort: baseline clinical features and comparison with other large scleroderma cohorts. Rheumatology 2018; 57: 1623–1631. [DOI] [PubMed] [Google Scholar]
- 23.Hunzelmann N, Genth E, Krieg T, et al. The registry of the German Network for Systemic Scleroderma: frequency of disease subsets and patterns of organ involvement. Rheumatology 2008; 47: 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer OC, Fertig N, Lucas M, et al. Disease subsets, antinuclear antibody profile, and clinical features in 127 French and 247 US adult patients with systemic sclerosis. J Rheumatol 2007; 34: 104–109. [PubMed] [Google Scholar]
- 25.Nihtyanova SI, Schreiber BE, Ong VH, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol 2014; 66: 1625–1635. [DOI] [PubMed] [Google Scholar]
- 26.Walker UA, Tyndall A, Czirják L, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Ann Rheum Dis 2007; 66: 754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann-Vold AM, Fretheim H, Halse AK, et al. Tracking impact of interstitial lung disease in systemic sclerosis in a complete nationwide cohort. Am J Respir Crit Care Med 2019; 200: 1258–1266. [DOI] [PubMed] [Google Scholar]
- 28.Mango RL, Matteson EL, Crowson CS, et al. Assessing mortality models in systemic sclerosis-related interstitial lung disease. Lung 2018; 196: 409–416. [DOI] [PubMed] [Google Scholar]
- 29.Rotondo C, Urso L, Praino E, et al. Thoracic lymphadenopathy as possible predictor of the onset of interstitial lung disease in systemic sclerosis patients without lung involvement at baseline visit: a retrospective analysis. J Scleroderma Rel Disord 2020; 5: 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010; 69: 1809–1815. [DOI] [PubMed] [Google Scholar]
- 31.Guler SA, Winstone TA, Murphy D, et al. Does systemic sclerosis-associated interstitial lung disease burn out? Specific phenotypes of disease progression. Ann Am Thorac Soc 2018; 15: 1427–1433. [DOI] [PubMed] [Google Scholar]
- 32.Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 2010; 62: 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carmona L, González-Alvaro I, Balsa A, et al. Rheumatoid arthritis in Spain: occurrence of extra-articular manifestations and estimates of disease severity. Ann Rheum Dis 2003; 62: 897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koduri G, Norton S, Young A, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology 2010; 49: 1483–1489. [DOI] [PubMed] [Google Scholar]
- 35.Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med 2011; 183: 372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richman NC, Yazdany J, Graf J, et al. Extraarticular manifestations of rheumatoid arthritis in a multiethnic cohort of predominantly Hispanic and Asian patients. Medicine 2013; 92: 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hyldgaard C, Hilberg O, Pedersen AB, et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis 2017; 76: 1700–1706. [DOI] [PubMed] [Google Scholar]
- 38.Kim EJ, Collard HR, King TE., Jr.Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest 2009; 136: 1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J, Shi Y, Wang X, et al. Asymptomatic preclinical rheumatoid arthritis-associated interstitial lung disease. Clin Dev Immunol 2013; 2013: 406927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly CA, Saravanan V, Nisar M, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics–a large multicentre UK study. Rheumatology 2014; 53: 1676–1682. [DOI] [PubMed] [Google Scholar]
- 41.Sparks JA, He X, Huang J, et al. Rheumatoid arthritis disease activity predicting incident clinically apparent rheumatoid arthritis–associated interstitial lung disease: a prospective cohort study. Arthritis Rheumatol 2019; 71: 1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyldgaard C, Ellingsen T, Hilberg O, et al. Rheumatoid arthritis-associated interstitial lung disease: clinical characteristics and predictors of mortality. Respiration 2019; 98: 455–460. [DOI] [PubMed] [Google Scholar]
- 43.Raimundo K, Solomon JJ, Olson AL, et al. Rheumatoid arthritis–interstitial lung disease in the United States: prevalence, incidence, and healthcare costs and mortality. J Rheumatol 2019; 46: 360–369. [DOI] [PubMed] [Google Scholar]
- 44.Chen IJ, Jan Wu YJ, Lin CW, et al. Interstitial lung disease in polymyositis and dermatomyositis. Clin Rheumatol 2009; 28: 639–646. [DOI] [PubMed] [Google Scholar]
- 45.Kang EH, Lee EB, Shin KC, et al. Interstitial lung disease in patients with polymyositis, dermatomyositis and amyopathic dermatomyositis. Rheumatology 2005; 44: 1282–1286. [DOI] [PubMed] [Google Scholar]
- 46.Marie I, Hachulla E, Chérin P, et al. Interstitial lung disease in polymyositis and dermatomyositis. Arthritis Rheum 2002; 47: 614–622. [DOI] [PubMed] [Google Scholar]
- 47.Lilleker JB, Vencovsky J, Wang G, et al. The EuroMyositis registry: an international collaborative tool to facilitate myositis research. Ann Rheum Dis 2018; 77: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dugar M, Cox S, Limaye V, et al. Clinical heterogeneity and prognostic features of South Australian patients with anti-synthetase autoantibodies. Intern Med J 2011; 41: 674–679. [DOI] [PubMed] [Google Scholar]
- 49.Hamaguchi Y, Fujimoto M, Matsushita T, et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS ONE 2013; 8: e60442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cottin V, Thivolet-Béjui F, Reynaud-Gaubert M, et al. Interstitial lung disease in amyopathic dermatomyositis, dermatomyositis and polymyositis. Eur Respir J 2003; 22: 245–250. [DOI] [PubMed] [Google Scholar]
- 51.Douglas WW, Tazelaar HD, Hartman TE, et al. Polymyositis-dermatomyositis-associated interstitial lung disease. Am J Respir Crit Care Med 2001; 164: 1182–1185. [DOI] [PubMed] [Google Scholar]
- 52.Fujisawa T, Hozumi H, Kono M, et al. Prognostic factors for myositis-associated interstitial lung disease. PLoS ONE 2014; 9: e98824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Z, Cao M, Plana MN, et al. Utility of anti–melanoma differentiation–associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res 2013; 65: 1316–1324. [DOI] [PubMed] [Google Scholar]
- 54.Hall JC, Casciola-Rosen L, Samedy LA, et al. Anti–melanoma differentiation–associated protein 5–associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res 2013; 65: 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X, Xu B, Ma Y, et al. Clinical and laboratory profiles of primary Sjogren’s syndrome in a Chinese population: a retrospective analysis of 315 patients. Int J Rheum Dis 2015; 18: 439–446. [DOI] [PubMed] [Google Scholar]
- 56.Manfredi A, Sebastiani M, Cerri S, et al. Prevalence and characterization of non-sicca onset primary Sjögren syndrome with interstitial lung involvement. Clin Rheumatol 2017; 36: 1261–1268. [DOI] [PubMed] [Google Scholar]
- 57.Nannini C, Jebakumar AJ, Crowson CS, et al. Primary Sjögren’s syndrome 1976–2005 and associated interstitial lung disease: a population-based study of incidence and mortality. BMJ Open 2013; 3: e003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sogkas G, Hirsch S, Olsson KM, et al. Lung involvement in Primary Sjögren’s syndrome-an under-diagnosed entity. Front Med 2020; 7: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong X, Zhou J, Guo X, et al. A retrospective analysis of distinguishing features of chest HRCT and clinical manifestation in primary Sjögren’s syndrome-related interstitial lung disease in a Chinese population. Clin Rheumatol 2018; 37: 2981–2988. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Hou Z, Qiu M, et al. Risk factors for primary Sjögren syndrome-associated interstitial lung disease. J Thorac Dis 2018; 10: 2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buvry C, Cassagnes L, Tekath M, et al. Anti-Ro52 antibodies are a risk factor for interstitial lung disease in primary Sjögren syndrome. Respir Med 2020; 163: 105895. [DOI] [PubMed] [Google Scholar]
- 62.Gao H, Zhang XW, He J, et al. Prevalence, risk factors, and prognosis of interstitial lung disease in a large cohort of Chinese primary Sjögren syndrome patients: a case-control study. Medicine 2018; 97: e11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao H, Zou YD, Zhang XW, et al. Interstitial lung disease in non-sicca onset primary Sjögren’s syndrome: a large-scale case-control study. Int J Rheum Dis 2018; 21: 1423–1429. [DOI] [PubMed] [Google Scholar]
- 64.He C, Chen Z, Liu S, et al. Prevalence and risk factors of interstitial lung disease in patients with primary Sjögren’s syndrome: a systematic review and meta-analysis. Int J Rheum Dis 2020; 23: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 65.Ito I, Nagai S, Kitaichi M, et al. Pulmonary manifestations of primary Sjogren’s syndrome: a clinical, radiologic, and pathologic study. Am J Respir Crit Care Med 2005; 171: 632–638. [DOI] [PubMed] [Google Scholar]
- 66.Parambil JG, Myers JL, Lindell RM, et al. Interstitial lung disease in primary Sjögren syndrome. Chest 2006; 130: 1489–1495. [DOI] [PubMed] [Google Scholar]
- 67.Ghosh A, Das T, Ghosh A, et al. Evaluation of respiratory manifestations in systemic lupus erythematosus with special reference to pulmonary interstitial involvement. J Indian Med Assoc 2012; 110: 109–111. [PubMed] [Google Scholar]
- 68.Jacobsen S, Petersen J, Ullman S, et al. A multicentre study of 513 Danish patients with systemic lupus erythematosus. I. Disease manifestations and analyses of clinical subsets. Clin Rheumatol 1998; 17: 468–477. [DOI] [PubMed] [Google Scholar]
- 69.Tanaka N, Kunihiro Y, Kubo M, et al. HRCT findings of collagen vascular disease-related interstitial pneumonia (CVD-IP): a comparative study among individual underlying diseases. Clin Radiol 2018; 73: 833.e1–833.e10. [DOI] [PubMed] [Google Scholar]
- 70.Toyoda Y, Koyama K, Kawano H, et al. Clinical features of interstitial pneumonia associated with systemic lupus erythematosus. Respir Investig 2019; 57: 435–443. [DOI] [PubMed] [Google Scholar]
- 71.Torre O, Harari S.Pleural and pulmonary involvement in systemic lupus erythematosus. Presse medicale 2011; 40: e19–e29. [DOI] [PubMed] [Google Scholar]
- 72.Enomoto N, Egashira R.Analysis of systemic lupus erythematosus-related interstitial pneumonia: a retrospective multicentre study 2019; 9: 7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arimura Y, Minoshima S, Tanaka U, et al. [Pulmonary involvement in patients with myeloperoxidase specific-antineutrophil cytoplasmic antibody]. Ryumachi 1995; 35: 46–55. [PubMed] [Google Scholar]
- 74.Arulkumaran N, Periselneris N, Gaskin G, et al. Interstitial lung disease and ANCA-associated vasculitis: a retrospective observational cohort study. Rheumatology 2011; 50: 2035–2043. [DOI] [PubMed] [Google Scholar]
- 75.Fernandez Casares M, Gonzalez A, Fielli M, et al. Microscopic polyangiitis associated with pulmonary fibrosis. Clin Rheumatol 2015; 34: 1273–1277. [DOI] [PubMed] [Google Scholar]
- 76.Hirayama K, Kobayashi M, Usui J, et al. Pulmonary involvements of anti-neutrophil cytoplasmic autoantibody-associated renal vasculitis in Japan. Nephrol Dial Transplant 2015; 30: i83–i93. [DOI] [PubMed] [Google Scholar]
- 77.Sada KE, Yamamura M, Harigai M, et al. Classification and characteristics of Japanese patients with antineutrophil cytoplasmic antibody-associated vasculitis in a nationwide, prospective, inception cohort study. Arthritis Res Ther 2014; 16: R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tzelepis GE, Kokosi M, Tzioufas A, et al. Prevalence and outcome of pulmonary fibrosis in microscopic polyangiitis. Eur Respir J 2010; 36: 116–121. [DOI] [PubMed] [Google Scholar]
- 79.Maillet T, Goletto T, Beltramo G, et al. Usual interstitial pneumonia in ANCA-associated vasculitis: a poor prognostic factor. J Autoimmun 2020; 106: 102338. [DOI] [PubMed] [Google Scholar]
- 80.Ahmad K, Barba T, Gamondes D, et al. Interstitial pneumonia with autoimmune features: clinical, radiologic, and histological characteristics and outcome in a series of 57 patients. Respir Med 2017; 123: 56–62. [DOI] [PubMed] [Google Scholar]
- 81.Chartrand S, Swigris JJ, Stanchev L, et al. Clinical features and natural history of interstitial pneumonia with autoimmune features: a single center experience. Respir Med 2016; 119: 150–154. [DOI] [PubMed] [Google Scholar]
- 82.Oldham JM, Adegunsoye A, Valenzi E, et al. Characterisation of patients with interstitial pneumonia with autoimmune features. Eur Respir J 2016; 47: 1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lim JU, Gil BM, Kang HS, et al. Interstitial pneumonia with autoimmune features show better survival and less exacerbations compared to idiopathic pulmonary fibrosis. BMC Pulm Med 2019; 19: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ng KH, Chen DY, Lin CH, et al. Risk of interstitial lung disease in patients with newly diagnosed systemic autoimmune rheumatic disease: a nationwide, population-based cohort study. Semin Arthritis Rheum 2020; 50: 840–845. [DOI] [PubMed] [Google Scholar]
- 85.Farrokh D, Abbasi B, Fallah-Rastegar Y, et al. The extrapulmonary manifestations of systemic sclerosis on chest high resolution computed tomography. Tanaffos 2015; 14: 193–200. [PMC free article] [PubMed] [Google Scholar]
- 86.Garber SJ, Wells AU, duBois RM, et al. Enlarged mediastinal lymph nodes in the fibrosing alveolitis of systemic sclerosis. Br J Radiol 1992; 65: 983–986. [DOI] [PubMed] [Google Scholar]
- 87.Wechsler RJ, Steiner RM, Spirn PW, et al. The relationship of thoracic lymphadenopathy to pulmonary interstitial disease in diffuse and limited systemic sclerosis: CT findings. AJR Am J Roentgenol 1996; 167: 101–104. [DOI] [PubMed] [Google Scholar]
- 88.Behr J, Furst DE.Pulmonary function tests. Rheumatology 2008; 47: v65–v67. [DOI] [PubMed] [Google Scholar]
- 89.Man A, Davidyock T, Ferguson LT, et al. Changes in forced vital capacity over time in systemic sclerosis: application of group-based trajectory modelling. Rheumatology 2015; 54: 1464–1471. [DOI] [PubMed] [Google Scholar]
- 90.Hoffmann-Vold AM, Allanore Y, Alves M, et al. Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann Rheum Dis 2021; 80: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Steen VD, Medsger TA., Jr.Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43: 2437–2444. [DOI] [PubMed] [Google Scholar]
- 92.Jaeger VK, Wirz EG, Allanore Y, et al. Incidences and risk factors of organ manifestations in the early course of systemic sclerosis: a longitudinal EUSTAR study. PLoS ONE 2016; 11: e0163894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khanna D, Tseng CH, Farmani N, et al. Clinical course of lung physiology in patients with scleroderma and interstitial lung disease: analysis of the Scleroderma Lung Study Placebo Group. Arthritis Rheum 2011; 63: 3078–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu W, Jordan S, Becker MO, et al. Prediction of progression of interstitial lung disease in patients with systemic sclerosis: the SPAR model. Ann Rheum Dis 2018; 77: 1326–1332. [DOI] [PubMed] [Google Scholar]
- 95.Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 2019; 381: 1718–1727. [DOI] [PubMed] [Google Scholar]
- 96.Steen VD, Medsger TA.Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis 2007; 66: 940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goh NS, Desai SR, Veeraraghavan S, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med 2008; 177: 1248–1254. [DOI] [PubMed] [Google Scholar]
- 98.Goh NS, Hoyles RK, Denton CP, et al. Short-term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol 2017; 69: 1670–1678. [DOI] [PubMed] [Google Scholar]
- 99.Volkmann ER, Tashkin DP, Sim M, et al. Short-term progression of interstitial lung disease in systemic sclerosis predicts long-term survival in two independent clinical trial cohorts. Ann Rheum Dis 2019; 78: 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morisset J, Vittinghoff E, Elicker BM, et al. Mortality risk prediction in scleroderma-related interstitial lung disease: the SADL model. Chest 2017; 152: 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoffmann-Vold AM, Aaløkken TM, Lund MB, et al. Predictive value of serial high-resolution computed tomography analyses and concurrent lung function tests in systemic sclerosis. Arthritis Rheumatol 2015; 67: 2205–2212. [DOI] [PubMed] [Google Scholar]
- 102.Showalter K, Hoffmann A, Rouleau G, et al. Performance of forced vital capacity and lung diffusion cutpoints for associated radiographic interstitial lung disease in systemic sclerosis. J Rheumatol 2018; 45: 1572–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Suliman YA, Dobrota R, Huscher D, et al. Brief report: pulmonary function tests: high rate of false-negative results in the early detection and screening of scleroderma-related interstitial lung disease. Arthritis Rheumatol 2015; 67: 3256–3261. [DOI] [PubMed] [Google Scholar]
- 104.Launay D, Remy-Jardin M, Michon-Pasturel U, et al. High resolution computed tomography in fibrosing alveolitis associated with systemic sclerosis. J Rheumatol 2006; 33: 1789–1801. [PubMed] [Google Scholar]
- 105.Hassan RI, Lubertino LI, Barth MA, et al. Lung ultrasound as a screening method for interstitial lung disease in patients with systemic sclerosis. J Clin Rheumatol 2019; 25: 304–307. [DOI] [PubMed] [Google Scholar]
- 106.Fairchild R, Chung M, Yang D, et al. Development and assessment of a novel lung ultrasound interpretation criteria for the detection of interstitial lung disease in systemic sclerosis. Arthritis Care Res. Epub ahead of print 31 May 2020. DOI: 10.1002/acr.24338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gutierrez M, Soto-Fajardo C, Pineda C, et al. Ultrasound in the assessment of interstitial lung disease in systemic sclerosis: a systematic literature review by the OMERACT ultrasound group. J Rheumatol 2020; 47: 991–1000. [DOI] [PubMed] [Google Scholar]
- 108.Chassagnon G, Martin C, Marini R, et al. Use of elastic registration in pulmonary MRI for the assessment of pulmonary fibrosis in patients with systemic sclerosis. Radiology 2019; 291: 487–492. [DOI] [PubMed] [Google Scholar]
- 109.Elhai M, Hoffmann-Vold AM, Avouac J, et al. Performance of candidate serum biomarkers for systemic sclerosis-associated interstitial lung disease. Arthritis Rheumatol 2019; 71: 972–982. [DOI] [PubMed] [Google Scholar]
- 110.Hoffmann-Vold A-M, Maher TM, Philpot EE, et al. The identification and management of interstitial lung disease in systemic sclerosis: evidence-based European consensus statements. Lancet Rheumatol 2020; 2: e71–e83. [DOI] [PubMed] [Google Scholar]
- 111.Gabbay E, Tarala R, Will R, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med 1997; 156: 528–535. [DOI] [PubMed] [Google Scholar]
- 112.Nurmi HM, Purokivi MK, Kärkkäinen MS, et al. Variable course of disease of rheumatoid arthritis-associated usual interstitial pneumonia compared to other subtypes. BMC Pulm Med 2016; 16: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zamora-Legoff JA, Krause ML, Crowson CS, et al. Progressive decline of lung function in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol 2017; 69: 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suda T, Kaida Y, Nakamura Y, et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir Med 2009; 103: 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hozumi H, Nakamura Y, Johkoh T, et al. Acute exacerbation in rheumatoid arthritis-associated interstitial lung disease: a retrospective case control study. BMJ Open 2013; 3: e003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim EJ, Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2010; 35: 1322–1328. [DOI] [PubMed] [Google Scholar]
- 117.Singh N, Varghese J, England BR, et al. Impact of the pattern of interstitial lung disease on mortality in rheumatoid arthritis: a systematic literature review and meta-analysis. Semin Arthritis Rheum 2019; 49: 358–365. [DOI] [PubMed] [Google Scholar]
- 118.Solomon JJ, Chung JH, Cosgrove GP, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2016; 47: 588–596. [DOI] [PubMed] [Google Scholar]
- 119.Zamora-Legoff JA, Krause ML, Crowson CS, et al. Patterns of interstitial lung disease and mortality in rheumatoid arthritis. Rheumatology 2017; 56: 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morisset J, Vittinghoff E, Lee BY, et al. The performance of the GAP model in patients with rheumatoid arthritis associated interstitial lung disease. Respir Med 2017; 127: 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Doyle TJ, Patel AS, Hatabu H, et al. Detection of rheumatoid arthritis–interstitial lung disease is enhanced by serum biomarkers. Am J Respir Crit Care Med 2015; 191: 1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Juge P-A, Lee JS, Ebstein E, et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med 2018; 379: 2209–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kinoshita F, Hamano H, Harada H, et al. Role of KL-6 in evaluating the disease severity of rheumatoid lung disease: comparison with HRCT. Respir Med 2004; 98: 1131–1137. [DOI] [PubMed] [Google Scholar]
- 124.Oguz EO, Kucuksahin O, Turgay M, et al. Association of serum KL-6 levels with interstitial lung disease in patients with connective tissue disease: a cross-sectional study. Clin Rheumatol 2016; 35: 663–666. [DOI] [PubMed] [Google Scholar]
- 125.England BR, Duryee MJ, Roul P, et al. Malondialdehyde–acetaldehyde adducts and antibody responses in rheumatoid arthritis–associated interstitial lung disease. Arthritis Rheumatol 2019; 71: 1483–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang T, Zheng XJ, Ji YL, et al. Tumour markers in rheumatoid arthritis-associated interstitial lung disease. Clin Exp Rheumatol 2016; 34: 587–591. [PubMed] [Google Scholar]
- 127.Moazedi-Fuerst FC, Kielhauser SM, Scheidl S, et al. Ultrasound screening for interstitial lung disease in rheumatoid arthritis. Clin Exp Rheumatol 2014; 32: 199–203. [PubMed] [Google Scholar]
- 128.Manfredi A, Cassone G, Cerri S, et al. Diagnostic accuracy of a velcro sound detector (VECTOR) for interstitial lung disease in rheumatoid arthritis patients: the InSPIRAtE validation study (INterStitial pneumonia in rheumatoid ArThritis with an electronic device). BMC Pulm Med 2019; 19: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fathi M, Vikgren J, Boijsen M, et al. Interstitial lung disease in polymyositis and dermatomyositis: longitudinal evaluation by pulmonary function and radiology. Arthritis Rheum 2008; 59: 677–685. [DOI] [PubMed] [Google Scholar]
- 130.Andersson H, Aaløkken TM, Günther A, et al. Pulmonary involvement in the antisynthetase syndrome: a comparative cross-sectional study. J Rheumatol 2016; 43: 1107–1113. [DOI] [PubMed] [Google Scholar]
- 131.Pinal-Fernandez I, Casal -Dominguez M, Huapaya JA, et al. A longitudinal cohort study of the anti-synthetase syndrome: increased severity of interstitial lung disease in black patients and patients with anti-PL7 and anti-PL12 autoantibodies. Rheumatology 2017; 56: 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Marie I, Hatron PY, Dominique S, et al. Short-term and long-term outcomes of interstitial lung disease in polymyositis and dermatomyositis: a series of 107 patients. Arthritis Rheum 2011; 63: 3439–3447. [DOI] [PubMed] [Google Scholar]
- 133.Yamakawa H, Hagiwara E, Kitamura H, et al. Predictive factors for the long-term deterioration of pulmonary function in interstitial lung disease associated with anti-aminoacyl-tRNA synthetase antibodies. Respiration 2018; 96: 210–221. [DOI] [PubMed] [Google Scholar]
- 134.Jablonski R, Bhorade S, Strek ME, et al. Recognition and management of myositis-associated rapidly progressive interstitial lung disease. Chest 2020; 158: 252–263. [DOI] [PubMed] [Google Scholar]
- 135.Kamiya H, Panlaqui OM, Izumi S, et al. Systematic review and meta-analysis of prognostic factors for idiopathic inflammatory myopathy-associated interstitial lung disease. BMJ Open 2018; 8: e023998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zamora AC, Hoskote SS, Abascal-Bolado B, et al. Clinical features and outcomes of interstitial lung disease in anti-Jo-1 positive antisynthetase syndrome. Respir Med 2016; 118: 39–45. [DOI] [PubMed] [Google Scholar]
- 137.Kampolis CF, Fragkioudaki S, Mavragani CP, et al. Prevalence and spectrum of symptomatic pulmonary involvement in primary Sjögren’s syndrome. Clin Exp Rheumatol 2018; 36: 94–101. [PubMed] [Google Scholar]
- 138.Roca F, Dominique S, Schmidt J, et al. Interstitial lung disease in primary Sjögren’s syndrome. Autoimmun Rev 2017; 16: 48–54. [DOI] [PubMed] [Google Scholar]
- 139.Zhang T, Yuan F, Xu L, et al. Characteristics of patients with primary Sjögren’s syndrome associated interstitial lung disease and relevant features of disease progression. Clin Rheumatol 2020; 39: 1561–1568. [DOI] [PubMed] [Google Scholar]
- 140.Manfredi A, Sebastiani M, Cerri S, et al. Acute exacerbation of interstitial lung diseases secondary to systemic rheumatic diseases: a prospective study and review of the literature. J Thorac Dis 2019; 11: 1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Enomoto Y, Takemura T, Hagiwara E, et al. Prognostic factors in interstitial lung disease associated with primary Sjögren’s syndrome: a retrospective analysis of 33 pathologically-proven cases. PLoS ONE 2013; 8: e73774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wan SA, Teh CL, Jobli AT.Lupus pneumonitis as the initial presentation of systemic lupus erythematosus: case series from a single institution. Lupus 2016; 25: 1485–1490. [DOI] [PubMed] [Google Scholar]
- 143.Haye Salinas MJ, Caeiro F, Saurit V, et al. Pleuropulmonary involvement in patients with systemic lupus erythematosus from a Latin American inception cohort (GLADEL). Lupus 2017; 26: 1368–1377. [DOI] [PubMed] [Google Scholar]
- 144.Comarmond C, Crestani B, Tazi A, et al. Pulmonary fibrosis in antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis: a series of 49 patients and review of the literature. Medicine 2014; 93: 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kwon M, Lee AS, Mira-Avendano I, et al. Interstitial lung disease in antineutrophil cytoplasmic antibody-associated vasculitis patients: comparison with idiopathic pulmonary fibrosis. J Clin Rheumatol. Epub ahead of print 31 March 2020. DOI: 10.1097/RHU.0000000000001357. [DOI] [PubMed] [Google Scholar]
- 146.Kagiyama N, Takayanagi N, Kanauchi T, et al. Antineutrophil cytoplasmic antibody-positive conversion and microscopic polyangiitis development in patients with idiopathic pulmonary fibrosis. BMJ Open Respir Res 2015; 2: e000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hozumi H, Oyama Y, Yasui H, et al. Clinical significance of myeloperoxidase-anti-neutrophil cytoplasmic antibody in idiopathic interstitial pneumonias. PLoS ONE 2018; 13: e0199659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Flores-Suárez LF, Ruiz N, Saldarriaga Rivera LM, et al. Reduced survival in microscopic polyangiitis patients with pulmonary fibrosis in a respiratory referral centre. Clin Rheumatol 2015; 34: 1653–1654. [DOI] [PubMed] [Google Scholar]
- 149.Schirmer JH, Wright MN, Vonthein R, et al. Clinical presentation and long-term outcome of 144 patients with microscopic polyangiitis in a monocentric German cohort. Rheumatology 2016; 55: 71–79. [DOI] [PubMed] [Google Scholar]
- 150.Kampolis CF, Venetsanopoulou AI, Karakontaki F, et al. How can autoantibodies predict the long-term outcome of patients with interstitial lung disease? Results from a retrospective cohort study. Autoimmun Rev 2018; 17: 1124–1133. [DOI] [PubMed] [Google Scholar]
- 151.Dai J, Wang L, Yan X, et al. Clinical features, risk factors, and outcomes of patients with interstitial pneumonia with autoimmune features: a population-based study. Clin Rheumatol 2018; 37: 2125–2132. [DOI] [PubMed] [Google Scholar]
- 152.Ito Y, Arita M, Kumagai S, et al. Serological and morphological prognostic factors in patients with interstitial pneumonia with autoimmune features. BMC Pulm Med 2017; 17: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sebastiani M, Cassone G, De Pasquale L, et al. Interstitial pneumonia with autoimmune features: a single center prospective follow-up study. Autoimmun Rev 2020; 19: 102451. [DOI] [PubMed] [Google Scholar]
- 154.Wang J, Zheng P, Huang Z, et al. Serum SP-A and KL-6 levels can predict the improvement and deterioration of patients with interstitial pneumonia with autoimmune features. BMC Pulm Med 2020; 20: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kameda M, Otsuka M, Chiba H, et al. CXCL9, CXCL10, and CXCL11; biomarkers of pulmonary inflammation associated with autoimmunity in patients with collagen vascular diseases–associated interstitial lung disease and interstitial pneumonia with autoimmune features. PLoS ONE 2020; 15: e0241719. [DOI] [PMC free article] [PubMed] [Google Scholar]