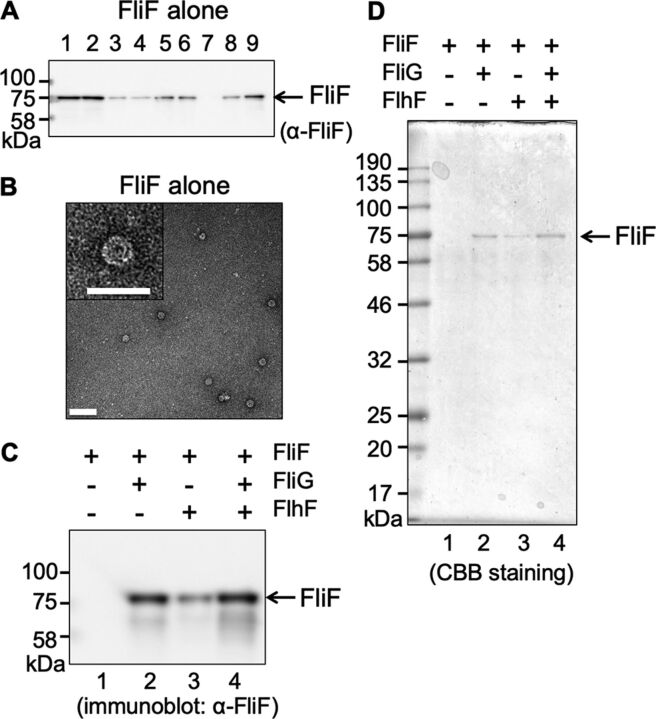
FIG 2.
MS-ring isolated from cells expressing FliF alone. (A) Immunoblot of the FliF protein during purification. Vibrio FliF was expressed in plasmid pRO101 in E. coli BL21(DE3) cells. Lane 1, suspension processed by the French press. Lane 2, supernatant from low-speed centrifugation. Lane 3, pellet from low-speed centrifugation. Lane 4, supernatant from ultracentrifugation (cytoplasmic fraction). Lane 5, pellet from ultracentrifugation (membrane fraction). Lane 6, supernatant from low-speed centrifugation following membrane solubilization by alkaline solution containing Triton X-100. Lane 7, pellet from low-speed centrifugation. Lane 8, supernatant from ultracentrifugation. Lane 9, pellet from ultracentrifugation (crude MS-ring fraction). (B) Electron microscopy (EM) image of purified MS-ring (bar, 100 nm). The right panel is an enlarged image of the MS-ring (bar, 50 nm). The grid was stained using uranyl acetate. (C) Immunoblot of the FliF protein contained in the MS-ring fraction (D) Coomassie brilliant blue-stained SDS-PAGE gel of the FliF protein contained in the MS-ring fraction. Lane 1, MS-ring fraction obtained from cells expressing Vibrio FliF alone in the pRO101 plasmid. Lane 2, MS-ring fraction obtained from cells coexpressing Vibrio FliF and FliG in the pTSK137 plasmid. Lane 3, MS-ring fraction obtained from cells coexpressing Vibrio FliF and FlhF in the pRO101 and pTSK122 plasmids, respectively. Lane 4, MS-ring fraction obtained from cells coexpressing Vibrio FliF, FliG, and FlhF in the pTSK137 and pTSK122 plasmids. The applied volume of the MS-ring samples was normalized by adjusting the culture absorbance at an optical density of 600 nm (OD600). All experiments were repeated more than twice, and the results were reproducible.