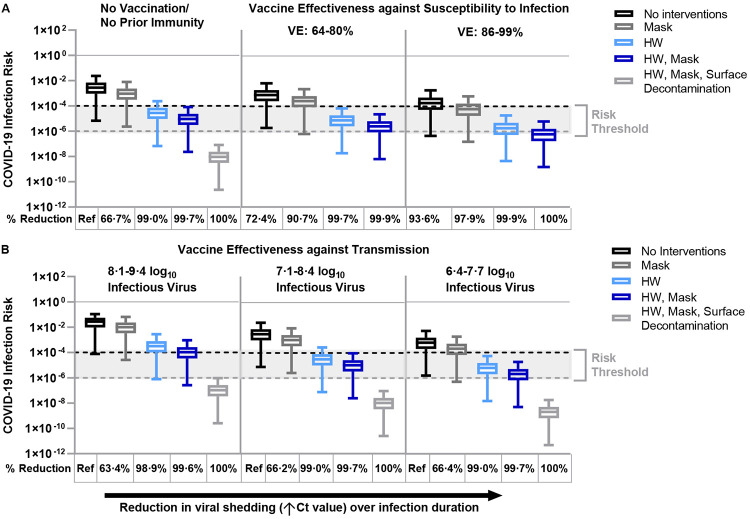
Figure 2.
Fomite-mediated SARS-CoV-2 infection risks associated with individual and combined standard infection control measures (hourly handwashing [2 log10 virus removal efficiency],58 surgical mask use). Vaccination was incorporated into the model representing two doses of mRNA vaccine (Moderna/Pfizer) and was applied with and without the standard infection control measures. Additional decontamination of plastic packaging [3 log10 virus removal efficiency]59 was applied in combination with the standard infection control measures. Ventilation (two air changes per hour [ACH]) was applied to all simulations. An infectious to non-infectious particle ratio of 1:10041 was applied to all viral shedding concentrations. Reductions in SARS-CoV-2 infection risk (%) to the susceptible worker relative to no interventions are reported below each panel. Panel A represents the impact of standard infection control measures with and without vaccination on fomite-mediated SARS-CoV-2 risk. For the first vaccination scenario, we assumed only the susceptible worker was vaccinated with two doses of mRNA vaccine (Moderna/Pfizer) and vaccine effectiveness (VE) against susceptibility to infection was simulated across three vaccination states. These included: 1) no vaccination/no prior immunity; 2) lower VE ranging from 6486-80%87 representative of reduced protection (variants of concern, waning immunity, immunocompromised and elderly or at-risk populations); and 3) optimal VE ranging from 86%88,89-9990% among healthy adults 14 days or more after second mRNA dose. Panel B: the second vaccine scenario represented vaccine effectiveness against transmission, where all workers are assumed to be vaccinated with two doses of mRNA vaccines and hence the model simulated rare breakthrough infections. Vaccine effectiveness against transmission (VET) was modeled by applying the combined effect of the reduction in risk of infection to the susceptible worker and the risk of transmissibility given a rare breakthrough infection among the vaccinated workers. We used the VET estimate (88·5% [95% CI: 82·3%, 94·8%] derived from Prunas et al.,62 VET was modeled across a range of three peak infectious viral shedding concentrations representative of possible increased transmissibility and/or infectiousness of variants of concern: 1) 8·1-9·4 log10 viral particles; 2) 7·1-8·4 log10 viral particles; and 3) 6·4-7·7 log10 viral particles. These viral shedding levels represent 100-, 10-, and 2-times, respectively, the increased viral shedding concentration simulated in the base model analysis. Dashed lines represent 1:10,000 (black) and 1:1,000,000 (grey) infection risk thresholds, derived from WHO and U.S. EPA guidelines for drinking water quality.60,61