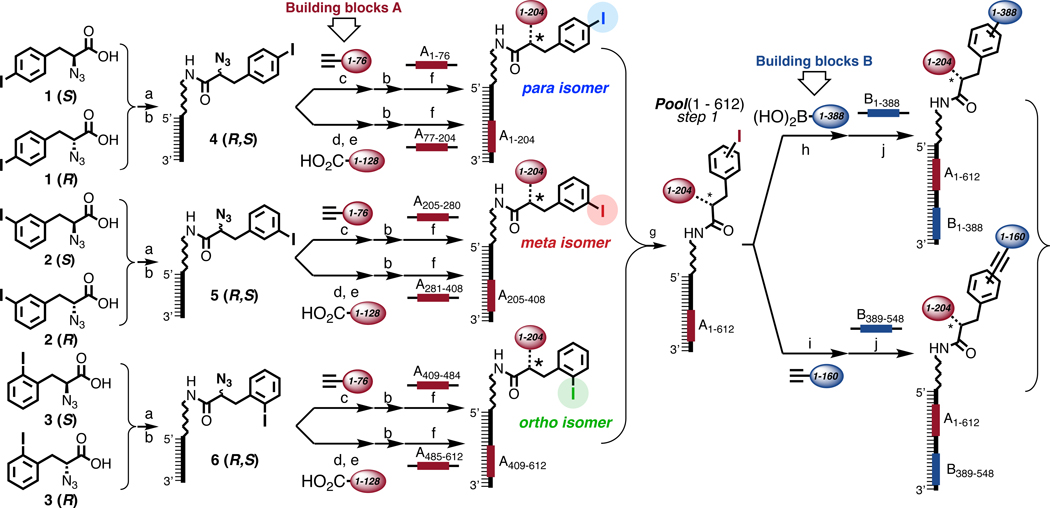
Figure 1 |. Library design, synthesis and encoding.
Schematic representation of the strategy used for library synthesis, using regio- and stereoisomers of 2-azido-3-iodophenylpropionic acid. The chemical building blocks A and B and the corresponding encoding DNA portions are color-coded in red and blue, respectively. The first set of building blocks was conjugated to the central scaffold through a triazole ring or amide bond (*), the second set of building blocks was connected by either Suzuki- or Sonogashira coupling. a, EDC, S-NHS, DIPEA, r.t., 30’, 5’-C6-amino-GGAGCTTCTGAATT in TEA buffer (pH=10), 37 °C, 6h. b, RP-HPLC purification. c, CuAAC on-DNA reaction37 d, TCEP, TRIS buffer pH=7, 40 °C, 3h, RP-HPLC purification. e, on-DNA amide bond formation85. f, adaptor 5’-CAGCACACAGAATTCAGAAGCTCC-3’, ligase buffer, T4 DNA-ligase. g, RP-HPLC purification at 60°C. h, Pd(OAc)2, TPPTS, 200 mM Na2CO3, 60°C, 3h37. i, Pd(OAc)2, TPPTS, CuSO4, Ascorbate, 200 mM Na2CO3, 70°C, 2h37. j, adaptor 5’- CGTCGATCCGGCGCCATGG-3’, ligase buffer, T4 DNA-ligase. See Chapter 4 and 8 of the Supplementary Information for exact structures and detailed conditions.