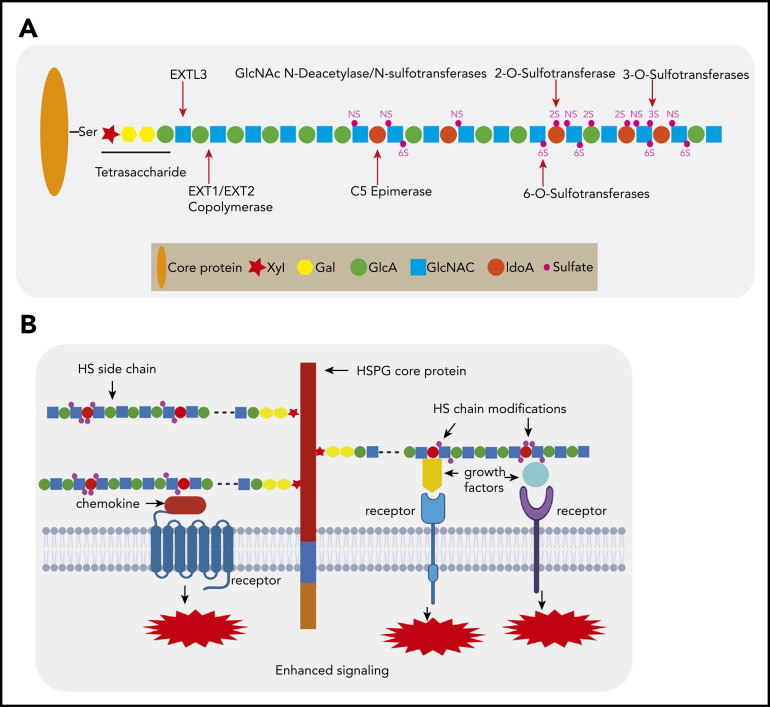
Figure 1.
HSPG structure and HS chain modification. (A) HSPGs consist of a core protein to which 1 or more HS side chains are covalently attached. The synthesis of a HS chain starts with the attachment of a tetrasaccharide linker (xylose–galactose–galactose–glucuronic acid) to a serine residue of the core protein. The enzyme EXTL3 adds the first GlcNAc to the tetrasaccharide linker. The co-polymerases EXT1/EXT2 mediate the elongation of the HS chain by adding GlcA-GlcNAc residues. Subsequently, the HS chains undergo various modifications, which include GlcNAc deacetylation and sulfation by N-deacetylase/N-sulfotransferases, epimerization of GlcA to its epimer iduronic acid by glucuronyl C5-epimerase, and sulfation at the 2-O-position, 3-O-position, and C-6-position by different sulfotransferases. After completion of this synthesis and modification process, the structure and function of the HS chain can still be further modified by a number of endo- and exo-enzymes. These include heparanase, which is expressed in damaged tissues and cancer but hardly by normal tissues and can cleave HS chains into fragments. Furthermore, the sulfation status of HS, which is crucial for protein interactions, can be modified by sulfatase-1 (Sulf-1) and sulfatase-2 (Sulf-2), which remove the 6-O sulfate moieties from disulfated GlcA-GlcNS6S and trisulfated IdoA2S-GlcNS6S. (B) HSPGs bind multiple growth factors, cytokines, and chemokines via their HS side chains and facilitate the interaction of these ligands with their cognate receptors, thereby regulating the activities of these proteins and leading to enhanced signaling activation. Importantly, HS modifications determine the binding capacity and specificity of HS chains for a given protein and are both cell type and differentiation stage specific.