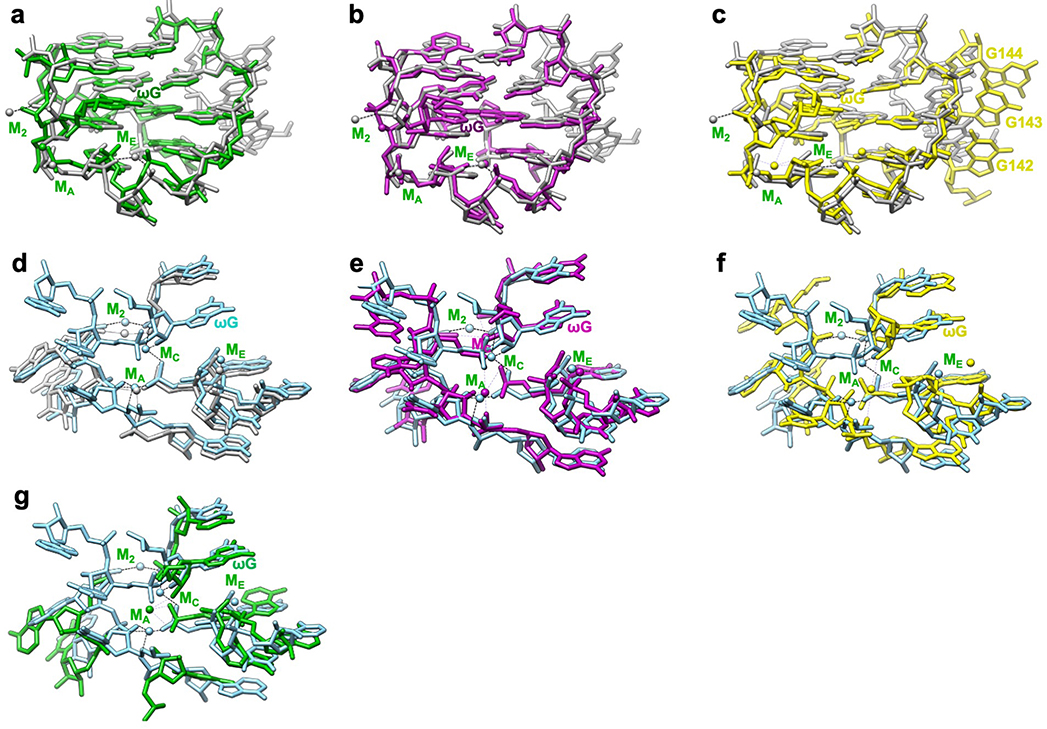
Extended Data Figure 8 related to Figure 4. Comparison of apo L-21 and holo L-16 ScaI ribozyme cryo-EM models with previous crystal structures show structural conservation and metal ion shifts in the guanosine binding site among group I introns.
The apo L-21 ScaI ribozyme adopts a preorganized guanosine binding site (grey) that superimposes with previous crystal structures of (a) mutated P3-P9 of the Tetrahymena ribozyme (green, PDB 1X8W), (b) Azoarcus ribozyme (violet, PDB 1U6B), and (c) phage Twort ribozyme (yellow, PDB 1y0q); The holo L-16 ScaI ribozyme (sky blue) superimposes with (d) apo L-21 ScaI ribozyme (grey), (e) Azoarcus ribozyme (violet), (f) mutated P3-P9 of the Tetrahymena ribozyme (green), and (g) phage Twort ribozyme (yellow). MC in the apo L-21 ScaI ribozyme is absent, whereas MC in Azoarcus ribozyme is shifted compared to the holo L-16 ScaI ribozyme. Dash line indicates metal ion coordination with surrounding atoms.